Hookworm infections: Reappraising the evidence for a role of neutrophils in light of NETosis
- PMID: 35124825
- PMCID: PMC9285577
- DOI: 10.1111/pim.12911
Hookworm infections: Reappraising the evidence for a role of neutrophils in light of NETosis
Abstract
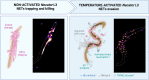
In Hookworm infection, neutrophils have long had the image of the villain, being recruited to the site of larval migration because of damage but participating themselves in tissue injury. With recent developments in neutrophil biology, there is an increasing body of evidence for the role of neutrophils as effector cells in hookworm immunity. In particular, their ability to release extracellular traps, or neutrophil extracellular traps (NETs), confer neutrophils a larvicidal activity. Here, we review recent evidence in this nascent field and discuss the avenue for future research on NETs/hookworm interactions.
Keywords: NETosis; helminth; hookworms; neutrophil extracellular traps; neutrophils.
© 2022 The Authors. Parasite Immunology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532‐1535. - PubMed
-
- Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12‐myristate 13‐acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J Leukoc Biol. 1996;59(2):229‐240. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

