ARID1A deficient undifferentiated spindle cell and rhabdoid sarcoma of the prostate: report of a unique case with emphasis on diagnostic implications
- PMID: 35125107
- PMCID: PMC8818209
- DOI: 10.1186/s13000-022-01198-4
ARID1A deficient undifferentiated spindle cell and rhabdoid sarcoma of the prostate: report of a unique case with emphasis on diagnostic implications
Abstract
Background: SWItch Sucrose Non-Fermentable (SWI/SNF) chromatin-remodeling complex functions collectively as a tumor suppressor and the inactivation of any of its constituent components is frequently associated with tumor initiation and/or progression. Most SWI/SNF deficient tumors share common rhabdoid morphology. ARID1A is the most frequently dysregulated SWI/SNF subunit in human cancer and inactivation of ARID1A is frequent across carcinomatous types while very rarely drives the tumorigenesis of sarcomas. Herein, we report a rare case of primary prostatic undifferentiated spindle cell sarcoma with focal rhabdoid morphology, harboring biallelic inactivation of ARID1A detected by next-generation sequencing with complete loss of ARID1A expression by immunohistochemistry.
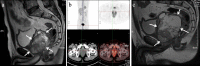
Case presentation: The patient is a 58-year-old man who presented with dysuria and obstructive voiding symptoms for 3 month and was found to have a large, ill-defined, prostatic mass lesion with circumferential extension into the rectal wall on imaging studies. A needle biopsy showed a spindle cell undifferentiated sarcoma of the prostate and the patient was treated by chemotherapy of combined etoposide and cisplatin for 2 months. A subsequent imaging study showed that the tumor was significantly enlarged, and the patient underwent laparoscopically radical prostatectomy. Gross examination showed a disrupted, 10 × 7 × 5 cm, solid and cystic mass involving almost the entire prostate and sparing the seminal vesicle glands. Histologic examination showed that tumor was composed mainly of mildly atypical, oval to spindle-shaped cells, arranged in sheets and fascicles or herringbone-like patterns within a small amount of edematous to myxoid, vascularized stroma. Notably, groups of discohesive rhabdoid tumor cells with eccentric nuclei, prominent nucleoli, and abundant globular cytoplasm were observed. There were prominent mitotic figures, multifocal geographic necroses, and foci of lymphovascular invasion. Immunohistochemistry showed that the tumor cells were diffusely positive for TLE-1 and vimentin and focally positive for epithelial membrane antigen, AE1/3, Cam5.2, SATB2, and CD34 (all in less than 10% tumor cells). Next-generation sequencing showed biallelic inactivation mutation of ARID1A; the predicted inactivating effect of ARID1A deletion was confirmed by immunohistochemical staining. After the surgery, the patient received an alternative combined chemotherapy of doxorubicin and ifosfamide for 5 months. The patient died 9 months after initial presentation due to extensive abdominal metastases.
Conclusions: We report an ARID1A deficient undifferentiated spindle cell and rhabdoid sarcoma of the prostate, adding to the growing spectrum of SWI/SNF driven undifferentiated sarcoma. Rhabdoid cells can be a helpful morphological clue for promoting molecular and immunohistochemical analyses for deficiency of SWI/SNF subunits, in the diagnostic workup of undifferentiated neoplasms featuring epithelioid or rhabdoid morphology.
Keywords: ARID1A; Biallelic inactivation; Case report; Prostate; Stromal sarcoma.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

