Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients
- PMID: 35125113
- PMCID: PMC8819971
- DOI: 10.1186/s12916-021-02230-y
Systematic review and meta-analysis of the diagnostic accuracy of prostate-specific antigen (PSA) for the detection of prostate cancer in symptomatic patients
Abstract
Background: Prostate-specific antigen (PSA) is a commonly used test to detect prostate cancer. Attention has mostly focused on the use of PSA in screening asymptomatic patients, but the diagnostic accuracy of PSA for prostate cancer in patients with symptoms is less well understood.
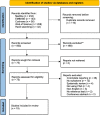
Methods: A systematic database search was conducted of Medline, EMBASE, Web of Science, and the Cochrane library. Studies reporting the diagnostic accuracy of PSA for prostate cancer in patients with symptoms were included. Two investigators independently assessed the titles and abstracts of all database search hits and full texts of potentially relevant studies against the inclusion criteria, and data extracted into a proforma. Study quality was assessed using the QUADAS-2 tool by two investigators independently. Summary estimates of diagnostic accuracy were calculated with meta-analysis using bivariate mixed effects regression.
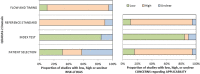
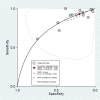
Results: Five hundred sixty-three search hits were assessed by title and abstract after de-duplication, with 75 full text papers reviewed. Nineteen studies met the inclusion criteria, 18 of which were conducted in secondary care settings with one from a screening study cohort. All studies used histology obtained by transrectal ultrasound-guided biopsy (TRUS) as a reference test; usually only for patients with elevated PSA or abnormal prostate examination. Pooled data from 14,489 patients found estimated sensitivity of PSA for prostate cancer was 0.93 (95% CI 0.88, 0.96) and specificity was 0.20 (95% CI 0.12, 0.33). The area under the hierarchical summary receiver operator characteristic curve was 0.72 (95% CI 0.68, 0.76). All studies were assessed as having a high risk of bias in at least one QUADAS-2 domain.
Conclusions: Currently available evidence suggests PSA is highly sensitive but poorly specific for prostate cancer detection in symptomatic patients. However, significant limitations in study design and reference test reduces the certainty of this estimate. There is very limited evidence for the performance of PSA in primary care, the healthcare setting where most PSA testing is performed.
Keywords: Diagnostic accuracy; LUTS; Lower urinary tract symptoms; PSA; Primary care; Prostate cancer; Prostate-specific antigen; Secondary care.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- NICE . Suspected cancer: recognition and referral. 2015. pp. 1–95. - PubMed
-
- Public Health England . Prostate Cancer Risk Management Programme (PCRMP): benefits and risks of PSA testing PCRMP. 2016.
-
- Mottet N, Cornford P, van den Bergh RCN. EAU, EANM, ESTRO, ESUR, ISUP, & SIOG. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous