Specific association of TBK1 with the trans-Golgi network following STING stimulation
- PMID: 35125375
- PMCID: PMC10511044
- DOI: 10.1247/csf.21080
Specific association of TBK1 with the trans-Golgi network following STING stimulation
Abstract
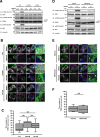
Stimulator of interferon genes (STING) is essential for the type I interferon response induced by microbial DNA or self-DNA leaked from mitochondria/nuclei. In response to the emergence of such DNAs in the cytosol, STING relocates from the endoplasmic reticulum (ER) to the Golgi, and activates TANK-binding kinase 1 (TBK1), a cytosolic kinase essential for the activation of STING-dependent downstream signalling. To understand at which subcellular compartments TBK1 becomes associated with STING, we generated cells stably expressing fluorescent protein-tagged STING (mNeonGreen-STING) and TBK1 (TBK1-mScarletI). We found that after STING stimulation, TBK1 became associated with the trans-Golgi network (TGN), not the other parts of the Golgi. STING variants that constitutively induce the type I interferon response have been identified in patients with autoinflammatory diseases named "STING-associated vasculopathy with onset in infancy (SAVI)". Even in cells expressing these constitutively active STING variants, TBK1 was found to be associated with TGN, not the other parts of the Golgi. These results suggest that TGN acts as a specific platform where STING associates with and activates TBK1.Key words: the Golgi, membrane traffic, innate immunity, STING.
Keywords: STING; innate immunity; membrane traffic; the Golgi.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Clark, K., Peggie, M., Plater, L., Sorcek, R.J., Young, E.R., Madwed, J.B., Hough, J., McIver, E.G., and Cohen, P.. 2011. Novel cross-talk within the IKK family controls innate immunity. Biochem. J., 434: 93–104. - PubMed
-
- Duran, J.M., Campelo, F., van Galen, J., Sachsenheimer, T., Sot, J., Egorov, M.V., Rentero, C., Enrich, C., Polishchuk, R.S., Goni, F.M., Brugger, B., Wieland, F., and Malhotra, V.. 2012. Sphingomyelin organization is required for vesicle biogenesis at the Golgi complex. EMBO J., 31: 4535–4546. - PMC - PubMed
-
- Fang, R., Jiang, Q., Guan, Y., Gao, P., Zhang, R., Zhao, Z., and Jiang, Z.. 2021. Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity, 54: 962–975.e8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

