Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway
- PMID: 35125823
- PMCID: PMC8793014
- DOI: 10.3748/wjg.v28.i1.123
Focal adhesion kinase-related non-kinase ameliorates liver fibrosis by inhibiting aerobic glycolysis via the FAK/Ras/c-myc/ENO1 pathway
Abstract
Background: Hepatic stellate cell (HSC) hyperactivation is a central link in liver fibrosis development. HSCs perform aerobic glycolysis to provide energy for their activation. Focal adhesion kinase (FAK) promotes aerobic glycolysis in cancer cells or fibroblasts, while FAK-related non-kinase (FRNK) inhibits FAK phosphorylation and biological functions.
Aim: To elucidate the effect of FRNK on liver fibrosis at the level of aerobic glycolytic metabolism in HSCs.
Methods: Mouse liver fibrosis models were established by administering CCl4, and the effect of FRNK on the degree of liver fibrosis in the model was evaluated. Transforming growth factor-β1 was used to activate LX-2 cells. Tyrosine phosphorylation at position 397 (pY397-FAK) was detected to identify activated FAK, and the expression of the glycolysis-related proteins monocarboxylate transporter 1 (MCT-1) and enolase1 (ENO1) was assessed. Bioinformatics analysis was performed to predict putative binding sites for c-myc in the ENO1 promoter region, which were validated with chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assays.
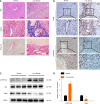
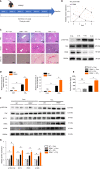
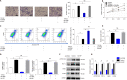
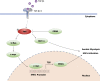
Results: The pY397-FAK level was increased in human fibrotic liver tissue. FRNK knockout promoted liver fibrosis in mouse models. It also increased the activation, migration, proliferation and aerobic glycolysis of primary hepatic stellate cells (pHSCs) but inhibited pHSC apoptosis. Nevertheless, opposite trends for these phenomena were observed after exogenous FRNK treatment in LX-2 cells. Mechanistically, the FAK/Ras/c-myc/ENO1 pathway promoted aerobic glycolysis, which was inhibited by exogenous FRNK.
Conclusion: FRNK inhibits aerobic glycolysis in HSCs by inhibiting the FAK/Ras/c-myc/ENO1 pathway, thereby improving liver fibrosis. FRNK might be a potential target for liver fibrosis treatment.
Keywords: Aerobic glycolysis; Enolase1; Focal adhesion kinase; Focal adhesion kinase-related non-kinase; Hepatic stellate cells; Liver fibrosis.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

