Targeting Sensory and Motor Integration for Recovery of Movement After CNS Injury
- PMID: 35126040
- PMCID: PMC8813971
- DOI: 10.3389/fnins.2021.791824
Targeting Sensory and Motor Integration for Recovery of Movement After CNS Injury
Abstract
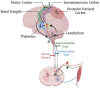
The central nervous system (CNS) integrates sensory and motor information to acquire skilled movements, known as sensory-motor integration (SMI). The reciprocal interaction of the sensory and motor systems is a prerequisite for learning and performing skilled movement. Injury to various nodes of the sensorimotor network causes impairment in movement execution and learning. Stimulation methods have been developed to directly recruit the sensorimotor system and modulate neural networks to restore movement after CNS injury. Part 1 reviews the main processes and anatomical interactions responsible for SMI in health. Part 2 details the effects of injury on sites critical for SMI, including the spinal cord, cerebellum, and cerebral cortex. Finally, Part 3 reviews the application of activity-dependent plasticity in ways that specifically target integration of sensory and motor systems. Understanding of each of these components is needed to advance strategies targeting SMI to improve rehabilitation in humans after injury.
Keywords: motor cortex; movement recovery; paired stimulation; plasticity; sensorimotor integration (SMI); spinal cord.
Copyright © 2022 Asan, McIntosh and Carmel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

