Antidepressant and Antipsychotic Drugs Reduce Viral Infection by SARS-CoV-2 and Fluoxetine Shows Antiviral Activity Against the Novel Variants in vitro
- PMID: 35126106
- PMCID: PMC8809408
- DOI: 10.3389/fphar.2021.755600
Antidepressant and Antipsychotic Drugs Reduce Viral Infection by SARS-CoV-2 and Fluoxetine Shows Antiviral Activity Against the Novel Variants in vitro
Abstract
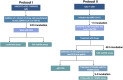
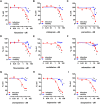
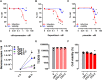
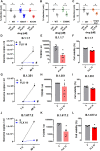
Repurposing of currently available drugs is a valuable strategy to tackle the consequences of COVID-19. Recently, several studies have investigated the effect of psychoactive drugs on SARS-CoV-2 in cell culture models as well as in clinical practice. Our aim was to expand these studies and test some of these compounds against newly emerged variants. Several antidepressants and antipsychotic drugs with different primary mechanisms of action were tested in ACE2/TMPRSS2-expressing human embryonic kidney cells against the infection by SARS-CoV-2 spike protein-dependent pseudoviruses. Some of these compounds were also tested in human lung epithelial cell line, Calu-1, against the first wave (B.1) lineage of SARS-CoV-2 and the variants of concern, B.1.1.7, B.1.351, and B.1.617.2. Several clinically used antidepressants, including fluoxetine, citalopram, reboxetine, imipramine, as well as antipsychotic compounds chlorpromazine, flupenthixol, and pimozide inhibited the infection by pseudotyped viruses with minimal effects on cell viability. The antiviral action of several of these drugs was verified in Calu-1 cells against the B.1 lineage of SARS-CoV-2. By contrast, the anticonvulsant carbamazepine, and novel antidepressants ketamine, known as anesthetic at high doses, and its derivatives as well as MAO and phosphodiesterase inhibitors phenelzine and rolipram, respectively, showed no activity in the pseudovirus model. Furthermore, fluoxetine remained effective against pseudoviruses with common receptor binding domain mutations, N501Y, K417N, and E484K, as well as B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) variants of SARS-CoV-2. Our study confirms previous data and extends information on the repurposing of these drugs to counteract SARS-CoV-2 infection including different variants of concern, however, extensive clinical studies must be performed to confirm our in vitro findings.
Keywords: SARS-CoV-2; alpha variant; antidepressants (AD); beta variant; delta variant; fluoxetine.
Copyright © 2022 Fred, Kuivanen, Ugurlu, Casarotto, Levanov, Saksela, Vapalahti and Castrén.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

