Development and Testing of a Spray-Dried Tuberculosis Vaccine Candidate in a Mouse Model
- PMID: 35126135
- PMCID: PMC8814656
- DOI: 10.3389/fphar.2021.799034
Development and Testing of a Spray-Dried Tuberculosis Vaccine Candidate in a Mouse Model
Abstract
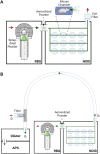
Converting a vaccine into a thermostable dry powder is advantageous as it reduces the resource burden linked with the cold chain and provides flexibility in dosage and administration through different routes. Such a dry powder presentation may be especially useful in the development of a vaccine towards the respiratory infectious disease tuberculosis (TB). This study assesses the immunogenicity and protective efficacy of spray-dried ID93+GLA-SE, a promising TB vaccine candidate, against Mycobacterium tuberculosis (Mtb) in a murine model when administered via different routes. Four administration routes for the spray-dried ID93+GLA-SE were evaluated along with relevant controls-1) reconstitution and intramuscular injection, 2) reconstitution and intranasal delivery, 3) nasal dry powder delivery via inhalation, and 4) pulmonary dry powder delivery via inhalation. Dry powder intranasal and pulmonary delivery was achieved using a custom nose-only inhalation device, and optimization using representative vaccine-free powder demonstrated that approximately 10 and 44% of the maximum possible delivered dose would be delivered for intranasal delivery and pulmonary delivery, respectively. Spray-dried powder was engineered according to the different administration routes including maintaining approximately equivalent delivered doses of ID93 and GLA. Vaccine properties of the different spray-dried lots were assessed for quality control in terms of nanoemulsion droplet diameter, polydispersity index, adjuvant content, and antigen content. Our results using the Mtb mouse challenge model show that both intranasal reconstituted vaccine delivery as well as pulmonary dry powder vaccine delivery resulted in Mtb control in infected mice comparable to traditional intramuscular delivery. Improved protection in these two vaccinated groups over their respective control groups coincided with the presence of cytokine-producing T cell responses. In summary, our results provide novel vaccine formulations and delivery routes that can be harnessed to provide protection against Mtb infection.
Keywords: ID93+GLA-SE; dry powder vaccine; in vivo murine model; nose-only inhalation device; particle engineering; respiratory delivery; tuberculosis; vaccine adjuvant formulation.
Copyright © 2022 Gomez, Ahmed, Das, McCollum, Mellett, Swanson, Gupta, Carrigy, Wang, Barona, Bachchhav, Gerhardt, Press, Archer, Liang, Seydoux, Kramer, Kuehl, Vehring, Khader and Fox.
Conflict of interest statement
MG, NC, MCA, RK, RV, and CF are inventors on a patent application involving spray-dried vaccine adjuvant compositions and methods, and CF is an inventor on a patent involving oil-in-water emulsions with low oil content including GLA-SE. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Aguilo N., Alvarez-Arguedas S., Uranga S., Marinova D., Monzón M., Badiola J., et al. (2015). Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17-Dependent Mechanism. J. Infect. Dis. 213, 831–839. 10.1093/infdis/jiv503 - DOI - PubMed
-
- Ahmed M., Smith D. M., Hamouda T., Rangel-Moreno J., Fattom A., Khader S. A. (2017). A Novel Nanoemulsion Vaccine Induces Mucosal Interleukin-17 Responses and Confers protection upon Mycobacterium tuberculosis challenge in Mice. Vaccine 35, 4983–4989. 10.1016/j.vaccine.2017.07.073 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

