Systems Pharmacology Modeling Identifies a Novel Treatment Strategy for Bortezomib-Induced Neuropathic Pain
- PMID: 35126148
- PMCID: PMC8809372
- DOI: 10.3389/fphar.2021.817236
Systems Pharmacology Modeling Identifies a Novel Treatment Strategy for Bortezomib-Induced Neuropathic Pain
Abstract
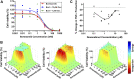
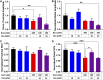
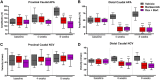
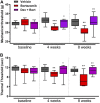
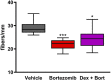
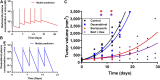
Chemotherapy-induced peripheral neurotoxicity is a common dose-limiting side effect of several cancer chemotherapeutic agents, and no effective therapies exist. Here we constructed a systems pharmacology model of intracellular signaling in peripheral neurons to identify novel drug targets for preventing peripheral neuropathy associated with proteasome inhibitors. Model predictions suggested the combinatorial inhibition of TNFα, NMDA receptors, and reactive oxygen species should prevent proteasome inhibitor-induced neuronal apoptosis. Dexanabinol, an inhibitor of all three targets, partially restored bortezomib-induced reduction of proximal action potential amplitude and distal nerve conduction velocity in vitro and prevented bortezomib-induced mechanical allodynia and thermal hyperalgesia in rats, including a partial recovery of intraepidermal nerve fiber density. Dexanabinol failed to restore bortezomib-induced decreases in electrophysiological endpoints in rats, and it did not compromise bortezomib anti-cancer effects in U266 multiple myeloma cells and a murine xenograft model. Owing to its favorable safety profile in humans and preclinical efficacy, dexanabinol might represent a treatment option for bortezomib-induced neuropathic pain.
Keywords: bortezomib; dexanabinol; multiple myeloma; peripheral neuropathy; pharmacodynamics; systems pharmacology.
Copyright © 2022 Bloomingdale, Meregalli, Pollard, Canta, Chiorazzi, Fumagalli, Monza, Pozzi, Alberti, Ballarini, Oggioni, Carlson, Liu, Ghandili, Ignatowski, Lee, Moore, Cavaletti and Mager.
Conflict of interest statement
MM is co-founder and CSO of AxoSim, Inc. DM is president and CEO of Enhanced Pharmacodynamics, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Antibody against tumor necrosis factor-α reduces bortezomib-induced allodynia in a rat model.Anticancer Res. 2013 Dec;33(12):5453-9. Anticancer Res. 2013. PMID: 24324081
-
Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord.Neuropharmacology. 2017 Sep 1;123:477-487. doi: 10.1016/j.neuropharm.2017.06.027. Epub 2017 Jun 27. Neuropharmacology. 2017. PMID: 28663117 Free PMC article.
-
High-dose intravenous immunoglobulins reduce nerve macrophage infiltration and the severity of bortezomib-induced peripheral neurotoxicity in rats.J Neuroinflammation. 2018 Aug 21;15(1):232. doi: 10.1186/s12974-018-1270-x. J Neuroinflammation. 2018. PMID: 30131066 Free PMC article.
-
Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy.Int J Mol Sci. 2021 Jan 17;22(2):888. doi: 10.3390/ijms22020888. Int J Mol Sci. 2021. PMID: 33477371 Free PMC article. Review.
-
Bortezomib and other proteosome inhibitors-induced peripheral neurotoxicity: From pathogenesis to treatment.J Peripher Nerv Syst. 2019 Oct;24 Suppl 2:S52-S62. doi: 10.1111/jns.12338. J Peripher Nerv Syst. 2019. PMID: 31647153 Review.
Cited by
-
Network pharmacology-based research on the effect of angelicin on osteosarcoma and the underlying mechanism.Aging (Albany NY). 2023 Jun 10;15(11):5125-5143. doi: 10.18632/aging.204786. Epub 2023 Jun 10. Aging (Albany NY). 2023. PMID: 37301545 Free PMC article.
-
Dynll1-PI31 Interaction Enhances Proteolysis Through the Proteasome, Representing a Novel Therapeutic Target for INF2-Related FSGS.Kidney360. 2025 Jan 1;6(1):38-48. doi: 10.34067/KID.0000000659. Epub 2024 Dec 2. Kidney360. 2025. PMID: 39621430 Free PMC article.
-
Recent advances in the treatment and prevention of peripheral neuropathy after multiple myeloma treatment.Ibrain. 2023 Sep 6;9(4):421-430. doi: 10.1002/ibra.12132. eCollection 2023 Winter. Ibrain. 2023. PMID: 38680507 Free PMC article. Review.
-
The nociceptive activity of peripheral sensory neurons is modulated by the neuronal membrane proteasome.Cell Rep. 2024 Apr 23;43(4):114058. doi: 10.1016/j.celrep.2024.114058. Epub 2024 Apr 12. Cell Rep. 2024. PMID: 38614084 Free PMC article.
References
LinkOut - more resources
Full Text Sources

