Expert Perspective: Who May Benefit Most From the New Ultra Long-Term Subcutaneous EEG Monitoring?
- PMID: 35126304
- PMCID: PMC8810530
- DOI: 10.3389/fneur.2021.817733
Expert Perspective: Who May Benefit Most From the New Ultra Long-Term Subcutaneous EEG Monitoring?
Abstract
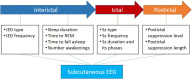
Today's modalities for short-term monitoring of EEG are primarily meant for supporting clinical diagnosis of epilepsy or classifying seizures and interictal epileptiform discharges while long-term EEG adds the value of differential diagnosis investigation or pre-surgical evaluation. However, longitudinal epilepsy care relies on patient diaries, which is known to be unreliable for most patients and especially those with focal impaired awareness or nocturnal seizures. The subcutaneous ultra long-term EEG (ULT-EEG) systems alleviate those issue by enabling objective, continuous EEG monitoring for days, weeks, months, or years. Albeit a great advance in continuous EEG over extended periods, it comes with the caveat of limited spatial resolution of two channels. Therefore, the new subcutaneous EEG modality may be especially suited for a selected group of patients. We convened a panel of experienced epileptologists to consider the utility of a subcutaneous, two-channel ULT-EEG device with the goal of developing a consensus-based expert recommendation on selecting the optimal patient types for this investigative technique. The ideal patients to select for this type of monitoring would have focal impaired awareness seizures without predominant motor features and seizures with medium to high voltage patterns. As this technology matures and we learn more about its limitations and benefits we might find a wider array of use case scenarios as it is believed that the benefits for many patients are most likely to outweigh the risks and cost.
Keywords: chronotherapy; circadian rhythm; epilepsy monitoring and recording; seizure detection; sub-scalp; subcutaneous EEG.
Copyright © 2022 Pathmanathan, Kjaer, Cole, Delanty, Surges and Duun-Henriksen.
Conflict of interest statement
All authors received a consultancy fee from UNEEG medical for the panel discussion. JP and TK consults for UNEEG medical. AC has received support from, and/or has served as a paid consultant for NeuroPace, Sage Therapeutics, and BrainVital/Precisis. ND has served on advisory boards and has received consultancy fees from UNEEG medical, Arvelle Therapeutics, Eisai, Sanofi, and UCB Pharma. RS reports lecture and consultancy fees from Angelini, Arvelle, Bial, Desitin, Eisai, LivaNova, Novartis, UCB Pharma, and UNEEG medical. JD-H is a full-time employee at UNEEG medical.
Figures

References
LinkOut - more resources
Full Text Sources
Medical
Research Materials

