Interkit Reproducibility of the Indirect Immunofluorescence Assay on HEp-2 Cells Depends on the Immunofluorescence Reactivity Intensity and Pattern
- PMID: 35126363
- PMCID: PMC8807640
- DOI: 10.3389/fimmu.2021.798322
Interkit Reproducibility of the Indirect Immunofluorescence Assay on HEp-2 Cells Depends on the Immunofluorescence Reactivity Intensity and Pattern
Abstract
Introduction: The indirect immunofluorescence assay on HEp-2 cells (HEp-2/IFA) is used worldwide for screening for autoantibodies to cellular antigens. Cell culture and fixation methods influence the cell distribution of autoantigens and the preservation of epitopes. Therefore, discrepancy of results obtained using different HEp-2/IFA kits (interkit nonreproducibility) is a common phenomenon in the clinical laboratory routine.
Objective: This study evaluated the interkit nonreproducibility of HEp-2/IFA results using samples from patients with systemic autoimmune disease (SAD), nonautoimmune diseases (NAD), and healthy blood donors (HBD).
Methods: Serum from 275 SAD patients, 293 NAD patients, and 300 HBD were processed at 1:80 dilution using four HEp-2 kits according to the manufacturers' instructions. Interkit reproducibility was determined for positive/negative results and patterns. The agreement of positive/negative results among kits for each sample was determined as the reactivity agreement score (RAS). The pattern reproducibility score (PRS) in each sample was calculated as a function of the number of kits showing equivalent patterns. Qualitative variables and ordinal variables were analyzed by the Chi-square and Mann-Whitney U tests, respectively.
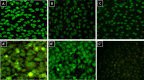
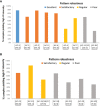
Results: A total of 402 samples were nonreactive in all kits and were considered devoid of autoantibodies. Further analysis included the 466 reactive samples (238 SAD, 119 NAD, 109 HBD). Reactivity to the nucleus had the highest interkit reproducibility (RAS = 83.6), followed by the metaphase plate (RAS = 78.9), cytoplasm (RAS = 77.4), and nucleolus (RAS = 72.4). Interkit reproducibility was higher in SAD (RAS = 78.0) than in NAD (RAS = 70.6) and HBD (RAS = 71.3) groups. Samples with strong reactivity (++++/4 and +++/4) had higher interkit reproducibility than those with weak reactivity (+/4). In the SAD group, RAS for nuclear reactivity was 87.5% for strongly reactive samples as opposed to 4.4% for weakly reactive samples, and the same was observed for NAD and HBD samples. The most robust patterns were the centromere AC-3 (PRS = 78.4), multiple nuclear dots AC-6 (PRS = 73.6), nuclear coarse speckled AC-5 (PRS = 71.3), nuclear homogeneous AC-1 (PRS = 67.9), and the reticular cytoplasmic AC-21 (PRS = 68.6).
Conclusion: Interkit nonreproducibility in HEp-2/IFA is prevalent and occurs with the highest frequency with weakly reactive samples. International initiatives with the engagement of in vitro diagnostic industry are encouraged to promote the harmonization of the properties and performance of HEp-2/IFA commercial kits.
Keywords: HEp-2 cells; antinuclear antibodies; autoantibody; autoimmune diseases; immunofluorescence.
Copyright © 2022 Silva, Dellavance, Baldo, Rodrigues, Grecco, Prado, Agustinelli and Andrade.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Mariz HA, Sato EI, Barbosa SH, Rodrigues SH, Dellavance A, Andrade LE. Pattern on the Antinuclear Antibody-HEp-2 Test Is a Critical Parameter for Discriminating Antinuclear Antibody-Positive Healthy Individuals and Patients With Autoimmune Rheumatic Diseases. Arthritis Rheum (2011) 63:191–200. doi: 10.1002/art.30084 - DOI - PubMed
-
- Dellavance A, Grabriel Junior A, Cintra AFU, Ximenes AC, Nuccitelli B, Taliberti BH, et al. . II Brazilian Consensus on Antinuclear Antibodies in HEp-2 Cells. Definitions for Standardization of Autoantibody Testing Against the Nucleus (ANA HEp-2), Nucleolus, Cytoplasm and Mitotic Apparatus, as Well as its Clinical Associations. Rev Rras Reumatol (2003) 43:129–40, ID:lil-386643.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

