Donor-But Not Recipient-Derived Cells Produce Collagen-1 in Chronically Rejected Cardiac Allografts
- PMID: 35126373
- PMCID: PMC8807636
- DOI: 10.3389/fimmu.2021.816509
Donor-But Not Recipient-Derived Cells Produce Collagen-1 in Chronically Rejected Cardiac Allografts
Abstract
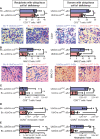
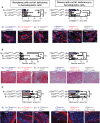
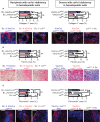
Fibrosis is a prominent feature of chronic allograft rejection, caused by an excessive production of matrix proteins, including collagen-1. Several cell types produce collagen-1, including mesenchymal fibroblasts and cells of hematopoietic origin. Here, we sought to determine whether tissue-resident donor-derived cells or allograft-infiltrating recipient-derived cells are responsible for allograft fibrosis, and whether hematopoietic cells contribute to collagen production. A fully MHC-mismatched mouse heterotopic heart transplantation model was used, with transient depletion of CD4+ T cells to prevent acute rejection. Collagen-1 was selectively knocked out in recipients or donors. In addition, collagen-1 was specifically deleted in hematopoietic cells. Tissue-resident macrophages were depleted using anti-CSF1R antibody. Allograft fibrosis and inflammation were quantified 20 days post-transplantation. Selective collagen-1 knock-out in recipients or donors showed that tissue-resident cells from donor hearts, but not infiltrating recipient-derived cells, are responsible for production of collagen-1 in allografts. Cell-type-specific knock-out experiments showed that hematopoietic tissue-resident cells in donor hearts substantially contributed to graft fibrosis. Tissue resident macrophages, however, were not responsible for collagen-production, as their deletion worsened allograft fibrosis. Donor-derived cells including those of hematopoietic origin determine allograft fibrosis, making them attractive targets for organ preconditioning to improve long-term transplantation outcomes.
Keywords: allograft fibrosis; chronic rejection; collagen-1; fibrocytes; transplantation.
Copyright © 2022 Balam, Buchtler, Winter, Schmidbauer, Neumayer, Talke, Renner, Geissler and Mack.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

