Neoantigens and their potential applications in tumor immunotherapy
- PMID: 35126730
- PMCID: PMC8805178
- DOI: 10.3892/ol.2022.13208
Neoantigens and their potential applications in tumor immunotherapy
Abstract
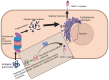
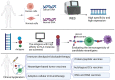
The incidence of malignant tumors is increasing, the majority of which are associated with high morbidity and mortality rates worldwide. The traditional treatment method for malignant tumors is surgery, coupled with radiotherapy or chemotherapy. However, these therapeutic strategies are frequently accompanied with adverse side effects. Over recent decades, tumor immunotherapy shown promise in demonstrating notable efficacy for the treatment of cancer. With the development of sequencing technology and bioinformatics algorithms, neoantigens have become compelling targets for cancer immunotherapy due to high levels of immunogenicity. In addition, neoantigen-based vaccines have demonstrated potential for cancer therapy, primarily by augmenting T-cell responses. Neoantigens have also been shown to be effective in immune checkpoint blockade therapy. Therefore, neoantigens may serve to be predictive biomarkers and synergistic treatment targets in cancer immunotherapy. The aim of the present review was to provide an overview of the recent progress in the classification, screening and clinical application of neoantigens for cancer therapy.
Keywords: immune checkpoint blockade; immunotherapy; neoantigen; tumor vaccine.
Copyright: © Fang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
