Small interfering RNA targeting N-cadherin regulates cell proliferation and migration in enzalutamide-resistant prostate cancer
- PMID: 35126732
- PMCID: PMC8805176
- DOI: 10.3892/ol.2022.13210
Small interfering RNA targeting N-cadherin regulates cell proliferation and migration in enzalutamide-resistant prostate cancer
Abstract
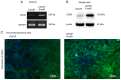
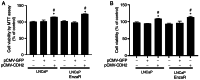
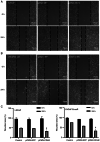
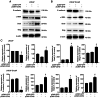
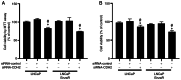
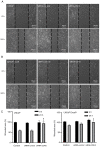
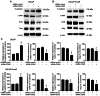
Enzalutamide is one of the options for treating patients with castration-resistant or metastatic prostate cancer. However, a substantial proportion of patients become resistant to enzalutamide after a period of treatment. Cells in these tumors typically exhibit increased proliferative and migratory capabilities, in which N-cadherin (CDH2) appear to serve an important role. In the present study, by up- and downregulating the expression of CDH2, the possible effects of CDH2 on the prostate cancer cell line LNCaP were investigated. Male sex hormone-sensitive LNCaP cells treated with 10 µM enzalutamide were named LNCaP enzalutamide-resistant (EnzaR) cells. Reverse transcription-PCR, western blotting and immunofluorescence staining were used to measure CDH2, E-cadherin, α-SMA, Snail and Slug expression. Transfection with the pCMV-CDH2 plasmid was performed for CDH2 upregulation, whilst transfection with small interfering RNA (siRNA)-CDH2 was performed for CDH2 downregulation. MTT and Cell Counting Kit-4 assays were used to evaluate the proportion of viable cancer cells. Subsequently, gap closure assay was performed to evaluate the migratory capability of both LNCaP and LNCaP EnzaR cell lines. CDH2 expression was found to be increased in LNCaP EnzaR cells compared with that in LNCaP cells. CDH2 overexpression increased cell viability and migration in both LNCaP and LNCaP EnzaR cell lines. By contrast, the opposite trend was observed after CDH2 expression was knocked down. CDH2 expression also showed a high association with that of four epithelial-mesenchymal transition markers, which was confirmed by western blotting. Based on these results, it was concluded that knocking down CDH2 expression using siRNA transfection mediated significant influence on LNCaP EnzaR cell physiology, which may be a potential therapeutic option for prostate cancer treatment.
Keywords: N-cadherin; enzalutamide resistance; migration; proliferation; prostate cancer.
Copyright: © Lu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
