Multivalent supramolecular assembly with ultralong organic room temperature phosphorescence, high transfer efficiency and ultrahigh antenna effect in water
- PMID: 35126989
- PMCID: PMC8730196
- DOI: 10.1039/d1sc05861d
Multivalent supramolecular assembly with ultralong organic room temperature phosphorescence, high transfer efficiency and ultrahigh antenna effect in water
Abstract
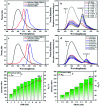
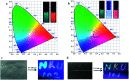
Multivalent supramolecular assemblies have recently attracted extensive attention in the applications of soft materials and cell imaging. Here, we report a novel multivalent supramolecular assembly constructed from 4-(4-bromophenyl)pyridine-1-ium bromide modified hyaluronic acid (HABr), cucurbit[8]uril (CB[8]) and laponite® clay (LP), which could emit purely organic room-temperature phosphorescence (RTP) with a phosphorescence lifetime of up to 4.79 ms in aqueous solution via multivalent supramolecular interactions. By doping the organic dyes rhodamine B (RhB) or sulfonated rhodamine 101 (SR101) into the HABr/CB[8]/LP assembly, phosphorescence energy transfer was realized with high transfer efficiency (energy transfer efficiency = 73-80%) and ultrahigh antenna effect (antenna effect value = 308-362) within the phosphorescent light harvesting system. Moreover, owing to the dynamic nature of the noncovalent interactions, a wide-range spectrum of phosphorescence energy transfer outputs could be obtained not only in water but also on filter paper and a glass plate by adjusting the donor-acceptor ratio and, importantly, white-light emission was obtained, which could be used in the application of information encryption.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

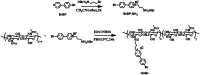


References
-
- Fateminia S. M. A. Mao Z. Xu S. Yang Z. Chi Z. Liu B. Angew. Chem., Int. Ed. 2017;56:12160–12164. doi: 10.1002/anie.201705945. - DOI - PubMed
- Yang J. Xu Z. Wang B. Gao X. Ren Z. Wang J. Xie Y. Li J. Peng Q. Pu K. Li Z. Nat. Commun. 2018;9:840. doi: 10.1038/s41467-018-03236-6. - DOI - PMC - PubMed
- Jia X. Shao C. Bai X. Zhou Q. Wu B. Wang L. Yue B. Zhu H. Zhu L. Proc. Natl. Acad. Sci. U. S. A. 2019;116:4816–4821. doi: 10.1073/pnas.1821991116. - DOI - PMC - PubMed
- Wang J. Huang Z. Ma X. Tian H. Angew. Chem., Int. Ed. 2020;59:9928–9933. doi: 10.1002/anie.201914513. - DOI - PubMed
- Liu X.-Q. Zhang K. Gao J.-F. Chen Y.-Z. Tung C.-H. Wu L.-Z. Angew. Chem., Int. Ed. 2020;59:23456–23460. doi: 10.1002/anie.202007039. - DOI - PubMed
- Dang Q. Jiang Y. Wang J. Wang J. Zhang Q. Zhang M. Luo S. Xie Y. Pu K. Li Q. Li Z. Adv. Mater. 2020;32:2006752. doi: 10.1002/adma.202006752. - DOI - PubMed
-
- Cai S. Shi H. Tian D. Ma H. Cheng Z. Wu Q. Gu M. Huang L. An Z. Peng Q. Huang W. Adv. Funct. Mater. 2018;28:1705045. doi: 10.1002/adfm.201705045. - DOI
- Su Y. Phua S. Z. F. Li Y. Zhou X. Jana D. Liu G. Lim W. Q. Ong W. K. Yang C. Zhao Y. Sci. Adv. 2018;4:eaas9732. doi: 10.1126/sciadv.aas9732. - DOI - PMC - PubMed
- Zhang X. Du L. Zhao W. Zhao Z. Xiong Y. He X. Gao P. F. Alam P. Wang C. Li Z. Leng J. Liu J. Zhou C. Lam J. W. Y. Phillips D. L. Zhang G. Tang B. Z. Nat. Commun. 2019;10:5161. doi: 10.1038/s41467-019-13048-x. - DOI - PMC - PubMed
- Yao X. Wang J. Jiao D. Huang Z. Mhirsi O. Lossada F. Chen L. Haehnle B. Kuehne A. J. C. Ma X. Tian H. Walther A. Adv. Mater. 2021;33:2005973. doi: 10.1002/adma.202005973. - DOI - PMC - PubMed
- Wu H. Chi W. Chen Z. Liu G. Gu L. Bindra A. K. Yang G. Liu X. Zhao Y. Adv. Funct. Mater. 2019;29:1807243. doi: 10.1002/adfm.201807243. - DOI
- Gu L. Wu H. Ma H. Ye W. Jia W. Wang H. Chen H. Zhang N. Wang D. Qian C. An Z. Huang W. Zhao Y. Nat. Commun. 2020;11:944. doi: 10.1038/s41467-020-14792-1. - DOI - PMC - PubMed
- Gu L. Ye W. Liang X. Lv A. Ma H. Singh M. Jia W. Shen Z. Guo Y. Gao Y. Chen H. Wang D. Wu Y. Liu J. Wang H. Zheng Y.-X. An Z. Huang W. Zhao Y. J. Am. Chem. Soc. 2021;143:18527–18535. doi: 10.1021/jacs.1c08118. - DOI - PubMed
- Yang J. Fang M. Li Z. Acc. Mater. Res. 2021;2:644–654. doi: 10.1021/accountsmr.1c00084. - DOI
-
- Zhang Q. Li B. Huang S. Nomura H. Tanaka H. Adachi C. Nat. Photonics. 2014;8:326–332. doi: 10.1038/nphoton.2014.12. - DOI
- Kabe R. Notsuka N. Yoshida K. Adachi C. Adv. Mater. 2016;28:655–660. doi: 10.1002/adma.201504321. - DOI - PubMed
- Li Q. Li Z. Acc. Chem. Res. 2020;53:962–973. doi: 10.1021/acs.accounts.0c00060. - DOI - PubMed
-
- Ma X. Wang J. Tian H. Acc. Chem. Res. 2019;52:738–748. doi: 10.1021/acs.accounts.8b00620. - DOI - PubMed
- Zhao W. He Z. Tang B. Z. Nat. Rev. Mater. 2020;5:869–885. doi: 10.1038/s41578-020-0223-z. - DOI
- Zhang T. Ma X. Wu H. Zhu L. Zhao Y. Tian H. Angew. Chem., Int. Ed. 2020;59:11206–11216. doi: 10.1002/anie.201915433. - DOI - PubMed
- Huang Z. Ma X. Cell Rep. Phys. Sci. 2020;1:100167. doi: 10.1016/j.xcrp.2020.100167. - DOI
-
- Datta S. Saha M. L. Stang P. J. Acc. Chem. Res. 2018;51:2047–2063. doi: 10.1021/acs.accounts.8b00233. - DOI - PMC - PubMed
- Ai Y. Chan M. H. Chan A. K. Ng M. Li Y. Yam V. W. Proc. Natl. Acad. Sci. U. S. A. 2019;116:13856–13861. doi: 10.1073/pnas.1908034116. - DOI - PMC - PubMed
- Zhang Y. Su Y. Wu H. Wang Z. Wang C. Zheng Y. Zheng X. Gao L. Zhou Q. Yang Y. Chen X. Yang C. Zhao Y. J. Am. Chem. Soc. 2021;143:13675–13685. doi: 10.1021/jacs.1c05213. - DOI - PubMed
- Zhou W.-L. Chen Y. Liu Y. Acta Chim. Sin. 2020;78:1164–1176. doi: 10.6023/A20100486. - DOI
- Zhou W.-L. Chen Y. Lin W. Liu Y. Chem. Commun. 2021;57:11443–11456. doi: 10.1039/D1CC04672A. - DOI - PubMed
- Zhou W. Chen Y. Yu Q. Li P. Chen X. Liu Y. Chem. Sci. 2019;10:3346–3352. doi: 10.1039/C9SC00026G. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

