Length-based separation of Bacillus subtilis bacterial populations by viscoelastic microfluidics
- PMID: 35127130
- PMCID: PMC8766588
- DOI: 10.1038/s41378-021-00333-3
Length-based separation of Bacillus subtilis bacterial populations by viscoelastic microfluidics
Abstract
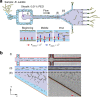
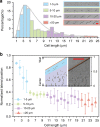
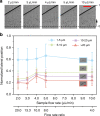
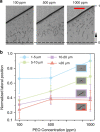
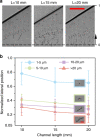
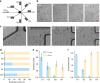
In this study, we demonstrated the label-free continuous separation and enrichment of Bacillus subtilis populations based on length using viscoelastic microfluidics. B. subtilis, a gram-positive, rod-shaped bacterium, has been widely used as a model organism and an industrial workhorse. B. subtilis can be arranged in different morphological forms, such as single rods, chains, and clumps, which reflect differences in cell types, phases of growth, genetic variation, and changing environmental factors. The ability to prepare B. subtilis populations with a uniform length is important for basic biological studies and efficient industrial applications. Here, we systematically investigated how flow rate ratio, poly(ethylene oxide) (PEO) concentration, and channel length affected the length-based separation of B. subtilis cells. The lateral positions of B. subtilis cells with varying morphologies in a straight rectangular microchannel were found to be dependent on cell length under the co-flow of viscoelastic and Newtonian fluids. Finally, we evaluated the ability of the viscoelastic microfluidic device to separate the two groups of B. subtilis cells by length (i.e., 1-5 μm and >5 μm) in terms of extraction purity (EP), extraction yield (EY), and enrichment factor (EF) and confirmed that the device could separate heterogeneous populations of bacteria using elasto-inertial effects.
Keywords: Chemistry; Engineering.
© The Author(s) 2022.
Conflict of interest statement
Conflict of interestThe authors declare no competing interests.
Figures
Similar articles
-
Separation and Enrichment of Yeast Saccharomyces cerevisiae by Shape Using Viscoelastic Microfluidics.Anal Chem. 2021 Jan 26;93(3):1586-1595. doi: 10.1021/acs.analchem.0c03990. Epub 2020 Dec 8. Anal Chem. 2021. PMID: 33289547
-
Microfluidic Separation and Enrichment of Escherichia coli by Size Using Viscoelastic Flows.Anal Chem. 2023 Jan 31;95(4):2561-2569. doi: 10.1021/acs.analchem.2c05084. Epub 2023 Jan 19. Anal Chem. 2023. PMID: 36656064
-
Ultra-Stretchable Microfluidic Devices for Optimizing Particle Manipulation in Viscoelastic Fluids.ACS Appl Mater Interfaces. 2024 Nov 13;16(45):61765-61773. doi: 10.1021/acsami.4c15893. Epub 2024 Nov 4. ACS Appl Mater Interfaces. 2024. PMID: 39496575
-
Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond.World J Microbiol Biotechnol. 2018 Sep 10;34(10):145. doi: 10.1007/s11274-018-2531-7. World J Microbiol Biotechnol. 2018. PMID: 30203131 Review.
-
Viscoelastic microfluidics: progress and challenges.Microsyst Nanoeng. 2020 Dec 14;6:113. doi: 10.1038/s41378-020-00218-x. eCollection 2020. Microsyst Nanoeng. 2020. PMID: 34567720 Free PMC article. Review.
Cited by
-
Exploitation of elasto-inertial fluid flow for the separation of nano-sized particles: Simulating the isolation of extracellular vesicles.Cytometry A. 2023 Oct;103(10):786-795. doi: 10.1002/cyto.a.24772. Epub 2023 Jun 23. Cytometry A. 2023. PMID: 37334483 Free PMC article.
-
Portable dielectrophoresis for biology: ADEPT facilitates cell trapping, separation, and interactions.Microsyst Nanoeng. 2024 Mar 1;10:29. doi: 10.1038/s41378-024-00654-z. eCollection 2024. Microsyst Nanoeng. 2024. PMID: 38434587 Free PMC article.
-
Separation of microalgae from bacterial contaminants using spiral microchannel in the presence of a chemoattractant.Bioresour Bioprocess. 2024 Apr 13;11(1):36. doi: 10.1186/s40643-024-00746-8. Bioresour Bioprocess. 2024. PMID: 38647805 Free PMC article.
-
A free-standing, phase-change liquid metal mold for 3D flexible microfluidics.Front Bioeng Biotechnol. 2022 Dec 5;10:1094294. doi: 10.3389/fbioe.2022.1094294. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36545676 Free PMC article.
-
Particle Separation in a Microchannel with a T-Shaped Cross-Section Using Co-Flow of Newtonian and Viscoelastic Fluids.Micromachines (Basel). 2023 Sep 28;14(10):1863. doi: 10.3390/mi14101863. Micromachines (Basel). 2023. PMID: 37893300 Free PMC article.
References
-
- Harwood CR. Bacillus subtilis and its relatives: Molecular biological and industrial workhorses. Trends Biotechnol. 1992;10:247–256. - PubMed
-
- Lopez D, Vlamakis H, Kolter R. Generation of multiple cell types in Bacillus subtilis. FEMS Microbiol. Rev. 2009;33:152–163. - PubMed
-
- Nordholt N, van Heerden JH, Bruggeman FJ. Biphasic cell-size and growth-rate homeostasis by single Bacillus subtilis cells. Curr. Biol. 2020;30:2238–2247.e2235. - PubMed
LinkOut - more resources
Full Text Sources
