Sevoflurane Aggravates the Progress of Alzheimer's Disease Through NLRP3/Caspase-1/Gasdermin D Pathway
- PMID: 35127716
- PMCID: PMC8807556
- DOI: 10.3389/fcell.2021.801422
Sevoflurane Aggravates the Progress of Alzheimer's Disease Through NLRP3/Caspase-1/Gasdermin D Pathway
Abstract
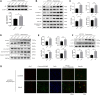
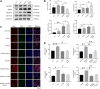
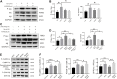
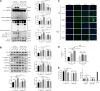
Background: Alzheimer's disease (AD) is the most common form of dementia worldwide. Previous studies have reported that sevoflurane, a frequently used anesthetic, can induce cognitive impairment in preclinical and clinical settings. However, the mechanism underlying the development of this neurotoxicity is currently unclear. Methods: Seven-month-old APP/PS1 mice were placed in an anesthesia induction box containing 3% sevoflurane in 100% O2 for 6 h, while BV2 cells were cultured with 4% sevoflurane for 6 h. Pyroptosis and tau protein expression in excised hippocampus tissues and cells were measured using Western blotting and immunofluorescence assay. Caspase-1 and NLRP3 were knocked out in BV2 microglia using CRISPR/Cas9 technology to determine whether they mediate the effects induced by sevoflurane. Results: Sevoflurane directly activated caspase-1 to induce pyroptosis in the mouse model of AD via NLRP3 and AIM2 activation. In addition, sevoflurane mediated cleavage of gasdermin D (GSDMD) but not gasdermin E (GSDME), promoted the biosynthesis of downstream interleukin-1β and interleukin-18, and increased β-amyloid (Aβ) deposition and tau phosphorylation. The nontoxic caspase-1 small-molecule inhibitor VX-765 significantly inhibited this activation process in microglia, while NLRP3 deletion suppressed sevoflurane-induced caspase-1 cleavage and subsequently pyroptosis, as well as tau pathology. Furthermore, silencing caspase-1 alleviated the sevoflurane-induced release of IL-1β and IL-18 and inhibited tau-related enzymes in microglia. Conclusion: This study is the first to report that clinical doses of sevoflurane aggravate the progression of AD via the NLRP3/caspase-1/GSDMD axis. Collectively, our findings elucidate the crucial mechanisms of NLRP3/caspase-1 in pyroptosis and tau pathogenesis induced by sevoflurane and suggest that VX-765 could represent a novel therapeutic intervention for treating AD.
Keywords: VX-765; gasdermin D; pyroptosis; sevoflurane; tau pathology.
Copyright © 2022 Tian, Xing, Gao, Zhang, Song, Tian and Dai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alfonso G., Carlo B., Giovanna P., Davide R., Valentina B., Cristina L., et al. (2018). Inflammation, Neurodegeneration and Protein Aggregation in the Retina as Ocular Biomarkers for Alzheimer's Disease in the 3xTg-AD Mouse Model. Cell Death Dis 9 (6), 685. 10.1038/s41419-018-0740-5 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

