Virological and Serological Characterisation of SARS-CoV-2 Infections Diagnosed After mRNA BNT162b2 Vaccination Between December 2020 and March 2021
- PMID: 35127770
- PMCID: PMC8810639
- DOI: 10.3389/fmed.2021.815870
Virological and Serological Characterisation of SARS-CoV-2 Infections Diagnosed After mRNA BNT162b2 Vaccination Between December 2020 and March 2021
Abstract
Background: Vaccines for coronavirus disease 2019 (COVID-19) are proving to be very effective in preventing severe illness; however, although rare, post-vaccine infections have been reported. The present study focuses on virological and serological features of 94 infections that occurred in Lazio Region (Central Italy) between 27 December 2020, and 30 March 2021, after one or two doses of mRNA BNT162b2 vaccine.
Methods: We evaluated clinical features, virological (viral load; viral infectiousness; genomic characterisation), and serological (anti-nucleoprotein Ig; anti-Spike RBD IgG; neutralising antibodies, nAb) characteristics of 94 post-vaccine infections at the time of diagnosis. Nasopharyngeal swabs (NPSs) and serum samples were collected in the framework of the surveillance activities on SARS-CoV-2 variants established in Lazio Region (Central Italy) and analysed at the National Institute for Infectious Diseases "L. Spallanzani" in Rome.
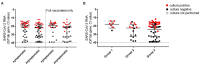
Results: The majority (92.6%) of the post-vaccine infections showed pauci/asymptomatic or mild clinical course, with symptoms and hospitalisation rate significantly less frequent in patients infected after full vaccination course as compared to patients who received a single dose vaccine. Although differences were not statistically significant, viral loads and isolation rates were lower in NPSs from patients infected after receiving two vaccine doses as compared to patients with one dose. Most cases (84%) had nAb in serum at the time of infection diagnosis, which is a sub-group of vaccinees, were found similarly able to neutralise Alpha and Gamma variants. Asymptomatic individuals showed higher nAb titres as compared to symptomatic cases (median titre: 1:120 vs. 1:40, respectively). Finally, the proportion of post-vaccine infections attributed either to Alpha and Gamma variants was similar to the proportion observed in the contemporary unvaccinated population in the Lazio region, and mutational analysis did not reveal enrichment of a defined set of Spike protein substitutions depending on the vaccination status.
Conclusion: Our study conducted using real-life data, emphasised the importance of monitoring vaccine breakthrough infections, through the characterisation of virological, immunological, and clinical features associated with these events, in order to tune prevention measures in the next phase of the COVID-19 pandemic.
Keywords: COVID-19; Italy; SARS-CoV-2; breakthrough infection; neutralising antibodies; vaccine; viral variants.
Copyright © 2022 Colavita, Meschi, Gruber, Rueca, Vairo, Matusali, Lapa, Giombini, De Carli, Spaziante, Messina, Bonfiglio, Carletti, Lalle, Fabeni, Berno, Puro, Bartolini, Di Caro, Ippolito, Capobianchi and Castilletti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. (2021) 397:1819–29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

