Elevated Serum D-Dimer May Reflect the Presence of Gut Inflammation in Spondyloarthritis
- PMID: 35127771
- PMCID: PMC8815704
- DOI: 10.3389/fmed.2021.816422
Elevated Serum D-Dimer May Reflect the Presence of Gut Inflammation in Spondyloarthritis
Abstract
Background: To investigate the association of D-dimer with gut inflammation in spondyloarthritis (SpA).
Methods: Sixty-five patients with SpA and 70 healthy controls were included. Demographic, clinical, and laboratory parameters were collected. The differences of clinical and laboratory parameters were compared between patients with SpA and healthy controls, and between patients with SpA, with and without gut inflammation. The associations of D-dimer with laboratory data were analyzed. The predictive value of D-dimer was obtained by a receiver operator characteristic (ROC) curve analysis. The independent risk factors for gut inflammation in SpA were investigated by binary logistic regression analysis.
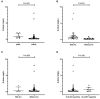
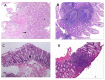
Results: Patients with SpA had higher D-dimer than healthy controls (P = 0.016). D-dimer was positively correlated with platelet (PLT), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), and negatively correlated with hemoglobin (Hb). Besides, significant differences were observed in D-dimer between SpA patients with and without gut inflammation (P < 0.001). Furthermore, SpA patients with gut inflammation were more likely to have peripheral joint involvement than those without gut inflammation (P < 0.001). The AUC of D-dimer was 0.865 at cut-off value of 0.29 mg/L, with a sensitivity of 82.6%, and a specificity of 81%. Elevated D-dimer (OR = 15.451, 95% CI: 3.030-78.780, P = 0.001) was independently associated with gut inflammation in SpA.
Conclusion: D-dimer may be a potential biomarker for identifying SpA patients with gut inflammation.
Keywords: d-dimer; gut inflammation; ileo-colonoscopy; peripheral joint involvement; spondyloarthritis.
Copyright © 2022 Feng, Li, Li, Jin, Du and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

