Paraoxonase-1 Activity in Breast Cancer Patients Treated With Doxorubicin With or Without Trastuzumab
- PMID: 35128203
- PMCID: PMC8807731
- DOI: 10.1016/j.jacbts.2021.10.010
Paraoxonase-1 Activity in Breast Cancer Patients Treated With Doxorubicin With or Without Trastuzumab
Abstract
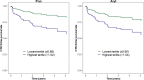
The objective of this study was to determine associations of paraoxonase-1 (PON-1) with development of cancer therapy-related cardiac dysfunction (CTRCD). PON-1 is a cardioprotective enzyme associated with high-density lipoprotein that prevents oxidized low-density lipoprotein formation. Given the role of oxidative stress in doxorubicin-induced cardiotoxicity, PON-1 activity may have relevance for the prediction of CTRCD. In 225 patients with breast cancer receiving doxorubicin with or without trastuzumab, we quantified PON-1 activity through its paraoxonase (Pon) and arylesterase (Aryl) enzymatic activity at baseline, during, and after doxorubicin completion. Echocardiograms were performed at baseline, during therapy, and annually. CTRCD was defined as a decrease in left ventricular ejection fraction by ≥10% from baseline to <50%. Associations between baseline biomarkers and clinical variables were determined using multivariable linear regression. Associations between changes in biomarker activity and time to CTRCD were evaluated using Cox regression. Pon was directly associated with Black race and inversely associated with Stage 2 cancer. Aryl was inversely associated with body mass index. After doxorubicin completion, activity levels of Pon and Aryl were significantly decreased (median ratio compared with baseline for Pon: 0.95 [Q1-Q3: 0.81-1.07, P < 0.001]; for Aryl: 0.97 [Q1-Q3: 0.85-1.08, P = 0.010]). A total of 184 patients had an available quantitated echocardiogram at baseline and at least 1 follow-up visit. Increases from baseline in Pon at doxorubicin completion were independently associated with increased CTRCD risk (per 10% increase: hazard ratio [HR]: 1.21; 95% confidence interval [CI]: 1.05-1.39; P = 0.007). Associations between increases in Aryl and CTRCD tended in the same direction but were of borderline statistical significance (HR: 1.17; 95% CI: 0.99-1.38; P = 0.071). In patients with breast cancer treated with doxorubicin with or without trastuzumab, increases in the Pon enzymatic activity level of PON-1 were associated with increased CTRCD risk. PON-1 activity may be relevant to mechanistic risk prediction of cardiotoxicity with anthracyclines.
Keywords: Aryl, arylesterase; BMI, body mass index; CTRCD, cancer therapy–related cardiac dysfunction; CVD, cardiovascular disease; HDL, high-density lipoprotein; HER2, human epidermal growth factor receptor 2; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; PON-1, paraoxonase-1; Pon, paraoxonase; cardiac dysfunction; cardiotoxicity; doxorubicin; heart failure; paraoxonase-1.
© 2022 Published by Elsevier on behalf of the American College of Cardiology Foundation.
Conflict of interest statement
Dr Fradley has received a research grant from Medtronic and has served as a consultant for Abbott and AstraZeneca, all unrelated to the contents of this paper. Dr Tang is a consultant for Sequana Medical A.G., Owkin Inc, Relypsa Inc, PreCardia Inc, and CardiolRx; and has received honoraria from Springer Nature for authorship/editorship and American Board of Internal Medicine for exam writing committee participation, all unrelated to the contents of this paper. Dr Ky has served as a consultant for Cytokinetics; and has received honoraria from Roche, unrelated to the contents of this paper. Ms Thompson is supported by a National Institutes of Health Medical Scientist Training Program Predoctoral T32 grant. For this work, she received additional support from the American Heart Association Student Scholarship in Cardiovascular Disease, the Perelman School of Medicine Center for Clinical Epidemiology and Biostatistics Summer Fellowship, and the Okun Family Cardiovascular Scholarship. This work is supported by R01HL118018 and R21HL141802, to Dr Ky. Dr Tang is partially supported by grants from the National Institutes of Health (R01HL126827). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- Harbeck N. Advances in targeting HER2-positive breast cancer. Curr Opin Obstet Gynecol. 2018;30:55–59. - PubMed
-
- Ahmed S., Sami A., Xiang J. HER2-directed therapy: current treatment options for HER2-positive breast cancer. Breast Cancer. 2015;22:101–116. - PubMed
-
- Slamon D.J., Godolphin W., Jones L.A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Madden R., Kosari S., Peterson G.M., Bagheri N., Thomas J. Lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer: a systematic review. Int J Clin Pharmacol Ther. 2018;56:72–80. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

