Translation of New and Emerging Therapies for Genetic Cardiomyopathies
- PMID: 35128211
- PMCID: PMC8807730
- DOI: 10.1016/j.jacbts.2021.07.012
Translation of New and Emerging Therapies for Genetic Cardiomyopathies
Abstract
The primary etiology of a diverse range of cardiomyopathies is now understood to be genetic, creating a new paradigm for targeting treatments on the basis of the underlying molecular cause. This review provides a genetic and etiologic context for the traditional clinical classifications of cardiomyopathy, including molecular subtypes that may exhibit differential responses to existing or emerging treatments. The authors describe several emerging cardiomyopathy treatments, including gene therapy, direct targeting of myofilament function, protein quality control, metabolism, and others. The authors discuss advantages and disadvantages of these approaches and indicate areas of high potential for short- and longer term efficacy.
Keywords: AAV, adeno-associated virus; ACM, arrhythmogenic cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; ATPase, adenosine triphosphatase; DCM, dilated cardiomyopathy; DMD, Duchenne muscular dystrophy; DNA, DNA; DSP, desmoplakin; FDA, U.S. Food and Drug Administration; GRT, gene replacement therapy; GST, gene silencing therapy; HCM, hypertrophic cardiomyopathy; HR, homologous recombination; LNP, lipid nanoparticle; LVOT, left ventricular outflow tract; RNA, RNA; TTR, transthyretin; arrhythmogenic cardiomyopathy; dilated cardiomyopathy; genetics; hypertrophic cardiomyopathy; therapeutics.
© 2022 The Authors.
Conflict of interest statement
Dr Day has received support from the National Heart, Lung, and Blood Institute (NHLBI) and Bristol Myers Squibb; and is a consultant for Bristol Myers Squibb, Tenaya Therapeutics, BioMarin Therapeutics, Lexeo Therapeutics, and Pfizer. Dr Helms has received support from the National Heart, Lung, and Blood Institute, the National Science Foundation, Tenaya Therapeutics, Lexeo Therapeutics, and Bristol Myers Squibb; and is a consultant for Tenaya Therapeutics. Dr Thompson has received support from the National Heart, Lung, and Blood Institute and Merck Manuals.
Figures
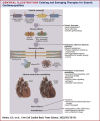



References
-
- Ko C., Arscott P., Concannon M., et al. Genetic testing impacts the utility of prospective familial screening in hypertrophic cardiomyopathy through identification of a nonfamilial subgroup. Genet Med. 2018;20:69–75. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

