Longitudinal changes of early motor and cognitive symptoms in progressive supranuclear palsy: the OxQUIP study
- PMID: 35128403
- PMCID: PMC8785161
- DOI: 10.1136/bmjno-2021-000214
Longitudinal changes of early motor and cognitive symptoms in progressive supranuclear palsy: the OxQUIP study
Abstract
Background: Progressive supranuclear palsy (PSP) is a rare neurodegenerative condition characterised by a range of motor and cognitive symptoms. Very little is known about the longitudinal change in these symptoms over time. Moreover, the effectiveness of clinical scales to detect early changes in PSP is still a matter of debate.
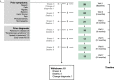
Objective: We aimed to determine longitudinal changes in PSP features using multiple closely spaced follow-up time points over a period of 2 years. Methods 28 healthy control and 28 PSP participants, with average time since onset of symptoms of 1.9 years, were prospectively studied every 3 months for up to 24 months. Changes from baseline scores were calculated at each follow-up time point using multiple clinical scales to identify longitudinal progression of motor and cognitive symptoms.
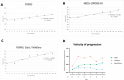
Results: The Montreal Cognitive Assessment, but not the Mini-Mental State Examination, detected cognitive decline at baseline. Both scales revealed poor longitudinal sensitivity to clinical change in global cognitive symptoms. Conversely, the Movement Disorders Society Unified Parkinson's disease Rating Scale - part III and the PSP Rating Scale (PSPRS) reliably detected motor decline less than 2 years after disease onset. The 'Gait/Midline' PSPRS subscore consistently declined over time, with the earliest change being observed 6 months after baseline assessment.
Conclusion: While better cognitive screening tools are still needed to monitor cognitive decline in PSP, motor decline is consistently captured by clinical rating scales. These results support the inclusion of multiple follow-up time points in longitudinal studies in the early stages of PSP.
Keywords: clinical neurology; motor control.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
