Polymer-templated mesoporous lithium titanate microspheres for high-performance lithium batteries
- PMID: 35128417
- PMCID: PMC8724910
- DOI: 10.1039/d1ma00708d
Polymer-templated mesoporous lithium titanate microspheres for high-performance lithium batteries
Abstract
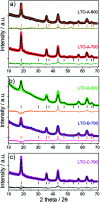
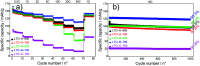
The spinel Li4Ti5O12 (LTO) is a promising lithium ion battery anode material with the potential to supplement graphite as an industry standard, but its low electrical conductivity and Li-ion diffusivity need to be overcome. Here, mesoporous LTO microspheres with carbon-coatings were formed by phase separation of a homopolymer from microphase-separated block copolymers of varying molar masses containing sol-gel precursors. Upon heating the composite underwent a sol-gel condensation reaction followed by the eventual pyrolysis of the polymer templates. The optimised mesoporous LTO microspheres demonstrated an excellent electrochemical performance with an excellent specific discharge capacity of 164 mA h g-1, 95% of which was retained after 1000 cycles at a C-rate of 10.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Zubi G. Dufo-Lopez R. Carvalho M. Pasaoglu G. Renewable Sustainable Energy Rev. 2018;89:292–308. doi: 10.1016/j.rser.2018.03.002. - DOI
-
- Ding Y. Cano Z. P. Yu A. Lu J. Chen Z. Electrochem. Energy Rev. 2019;2:1–28. doi: 10.1007/s41918-018-0022-z. - DOI
-
- Zhu G.-N. Wang Y.-G. Xia Y.-Y. Energy Environ. Sci. 2012;5:6652–6667. doi: 10.1039/C2EE03410G. - DOI
LinkOut - more resources
Full Text Sources
