Zebrafish stm is involved in the development of otoliths and of the fertilization envelope
- PMID: 35128429
- PMCID: PMC8812434
- DOI: 10.1530/RAF-20-0040
Zebrafish stm is involved in the development of otoliths and of the fertilization envelope
Abstract
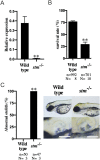
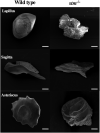
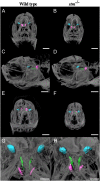
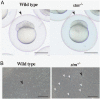
Using an in vivo assay, we selected 11 genes that were highly upregulated during the induction of ovulation in zebrafish using microarray analysis and RNA sequencing. The starmaker gene (stm) was one of these genes. Although stm has been previously reported to be involved in otolith formation during the early development of zebrafish, we detected its expression in eggs and showed that stm was related to fertilization by establishing an stm gene knockout strain using the CRISPR/Cas9 system. Further phenotypic analysis of stm knockout fish was conducted in this study. With a higher nonfertilization rate, the stm mutant strain showed an extremely low survival rate. Otoliths of stm homozygous mutant zebrafish showed abnormal morphology in embryos and adult fish. However, fish did not show any abnormalities in swimming behaviour in either embryos or adults. Stm proteins were detected on the chorion of ovulated eggs before spawning. Fibre-supported knob-like structures on the fertilization envelope (FE) also showed abnormal structures in stm mutants. The Stm protein is necessary for otolith formation, and a lack of Stm causes abnormal otolith formation. The partial defect of otolith formation does not cause defects in swimming behaviour. The Stm protein is expressed in the chorion and is responsible for the formation of fibre-supported knob-like structures on the FE. It was suggested that a lack of Stm caused a lower fertilization rate due to inadequate formation of the FE.
Lay summary: In zebrafish, the protein Starmaker (Stm) was identified as having a role in ovulation. Stm is also known to be required for the formation of ear stones (otoliths) which are needed to keep the body in balance. Zebrafish lacking Stm were produced by genome editing. As expected, Stm-deficient fish formed abnormal otoliths. To investigate the role of Stm in ovulation, fertilization and early development, we tried mating of Stm mutants and observed their juveniles. Although no problem found in ovulation, we found low fertilization rate and abnormal structure of knob-like structure (small pit) on the egg membrane. Survival rate of embryos with abnormal egg membrane was extremely low. It was demonstrated that Stm protein is necessary to form the functional egg membrane to protect embryos from the outside environment.
Keywords: fertilization; fertilization envelope; otoliths; starmaker; zebrafish.
© 2021 The authors.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Figures





Similar articles
-
Zebrafish prss59.1 is involved in chorion development.Gen Comp Endocrinol. 2024 Apr 1;349:114453. doi: 10.1016/j.ygcen.2024.114453. Epub 2024 Jan 27. Gen Comp Endocrinol. 2024. PMID: 38281702
-
In vivo and in vitro analysis of starmaker activity in zebrafish otolith biomineralization.FASEB J. 2019 Jun;33(6):6877-6886. doi: 10.1096/fj.201802268R. Epub 2019 Mar 6. FASEB J. 2019. PMID: 30840836
-
Candidate gene identification of ovulation-inducing genes by RNA sequencing with an in vivo assay in zebrafish.PLoS One. 2018 May 1;13(5):e0196544. doi: 10.1371/journal.pone.0196544. eCollection 2018. PLoS One. 2018. PMID: 29715317 Free PMC article.
-
Enigmatic ear stones: what we know about the functional role and evolution of fish otoliths.Biol Rev Camb Philos Soc. 2019 Apr;94(2):457-482. doi: 10.1111/brv.12463. Epub 2018 Sep 21. Biol Rev Camb Philos Soc. 2019. PMID: 30239135 Review.
-
Mechanisms of otoconia and otolith development.Dev Dyn. 2015 Mar;244(3):239-53. doi: 10.1002/dvdy.24195. Epub 2014 Oct 18. Dev Dyn. 2015. PMID: 25255879 Free PMC article. Review.
Cited by
-
Membrane progesterone receptor γ (paqr5b) is essential for the formation of neurons in the zebrafish olfactory rosette.Sci Rep. 2024 Oct 17;14(1):24354. doi: 10.1038/s41598-024-74674-0. Sci Rep. 2024. PMID: 39420013 Free PMC article.
-
In Search of a Target Gene for a Desirable Phenotype in Aquaculture: Genome Editing of Cyprinidae and Salmonidae Species.Genes (Basel). 2024 Jun 1;15(6):726. doi: 10.3390/genes15060726. Genes (Basel). 2024. PMID: 38927661 Free PMC article. Review.
-
Membrane progesterone receptor e (paqr9) is necessary for chorion elevation in zebrafish.Fish Physiol Biochem. 2025 Feb;51(1):1. doi: 10.1007/s10695-024-01435-1. Epub 2024 Dec 6. Fish Physiol Biochem. 2025. PMID: 39643858
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

