Efficacy and Safety of First-line Systemic Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis
- PMID: 35128482
- PMCID: PMC8792068
- DOI: 10.1016/j.euros.2021.12.007
Efficacy and Safety of First-line Systemic Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Network Meta-analysis
Abstract
Context: Considerable advances have been made in the first-line treatment of metastatic renal cell carcinoma (mRCC), with immunotherapy-based combinations including immunotherapy-tyrosine kinase inhibitors (IO-TKIs) and dual immunotherapy (IO-IO) favored. A lack of head-to-head clinical trials comparing these treatments means that there is uncertainty regarding their use in clinical practice.
Objective: To compare and rank the efficacy and safety of first-line systemic treatments for mRCC with a focus on IO-based combinations.
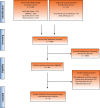
Evidence acquisition: MEDLINE (Ovid), EMBASE, Cochrane Library, Web of Science, and abstracts of recent major scientific meetings were searched to identify the most up-to-date phase 3 randomized controlled trials (RCTs) of first-line IO-based combinations for mRCC up to June 2021. A systematic review and network meta-analysis were completed using the Bayesian framework. Primary endpoints included overall survival (OS) and progression-free survival (PFS). Secondary endpoints included the objective response rate (ORR), complete response (CR), grade 3-4 treatment-related adverse events (TRAEs), treatment-related drug discontinuation (TRDD), and health-related quality of life (HRQoL). The analysis was performed for the intention-to-treat (ITT) population as well as by clinical risk group.
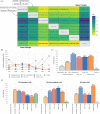
Evidence synthesis: A total of six phase 3 RCTs were included involving a total of 5121 patients. Nivolumab plus cabozantinib (NIVO-CABO) had the highest likelihood of an OS benefit in the ITT population (surface under the cumulative ranking curve 82%). Avelumab plus axitinib (AVEL-AXI) had the highest likelihood of an OS benefit for patients with favorable risk (65%). Pembrolizumab plus AXI (PEMBRO-AXI) had the highest likelihood of an OS benefit for patients with intermediate risk (78%). PEMBRO plus lenvatinib (PEMBRO-LENV) had the highest likelihood of an OS benefit for patients with poor risk (89%). PEMBRO-LENV was associated with a superior PFS benefit across all risk groups (89-98%). Maximal ORR was achieved with PEMBRO-LENV (97%). The highest likelihood for CR was attained with NIVO plus ipilimumab (NIVO-IPI; 85%) and PEMBRO-LENV (83%). The highest grade 3-4 TRAE rate occurred with PEMBRO-LENV (95%) and NIVO-CABO (83%), but the latter was associated with the lowest TRDD rate (2%). By contrast, NIVO-IPI had the lowest grade 3-4 TRAE rate (6%) and the highest likelihood of TRDD (100%). HRQoL consistently favored NIVO-CABO (66-75%), PEMBRO-LENV (44-85%), and NIVO-IPI (65-93%) in comparison to the other treatments.
Conclusions: IO-TKI drug combinations are associated with consistent improvements in clinically relevant outcomes for all mRCC risk groups. This benefit may be at the cost of higher TRAE rates; however, lower TRDD rates suggest a manageable side-effect profile. Longer follow-up is required to determine if the benefits of IO-TKIs will be sustained and if they should be favored in the first-line treatment of mRCC.
Patient summary: Combination treatments based on immunotherapy agents continue to show meaningful benefits in the first-line treatment of metastatic kidney cancer. Our review and network meta-analysis shows that immunotherapy combined with another class of agents called tyrosine kinase inhibitors is promising. However, longer follow-up is needed for this treatment strategy to clarify if the benefits are long-lasting.
Keywords: Immunotherapy; Network meta-analysis; Renal cell carcinoma; Tyrosine kinase inhibitors.
© 2022 The Authors.
Figures



References
-
- Surveillance, Epidemiology and End Results Program. SEER stat fact sheets: kidney and renal pelvis cancer. Bethesda, MD: National Cancer Institute; 2015. https://seer.cancer.gov/statfacts/html/kidrp.html.
Publication types
LinkOut - more resources
Full Text Sources

