Zinc deficiency impairs ischemia-induced angiogenesis
- PMID: 35128488
- PMCID: PMC8792263
- DOI: 10.1016/j.jvssci.2021.09.023
Zinc deficiency impairs ischemia-induced angiogenesis
Abstract
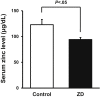
Objective: Zinc is an important essential trace metal involved in many physiologic functions, and its deficiency can affect the development of multiple organs, including the vasculature. However, clarity is lacking regarding the effects of zinc deficiency in the regulation of angiogenesis. We investigated the effects of zinc deficiency on the revascularization process through animal experiments and examined the relationship between the circulating zinc levels and tissue blood perfusion in patients with chronic limb-threatening ischemia (CLTI).
Methods: Zinc-deficient mice and control wild-type mice had undergone surgery to create unilateral hindlimb ischemia. Next, we examined the relationship between the serum zinc levels and skin perfusion pressure (SPP) as an index of tissue blood perfusion in patients with CLTI. A total of 51 patients with CLTI who had been referred for de novo revascularization for CLTI due to arteriosclerosis obliterans at our hospital from May 2012 to March 2016 were enrolled.
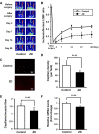
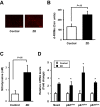
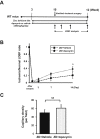
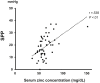
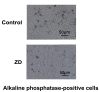
Results: The zinc-deficient mice showed a significant reduction in blood flow recovery rates in the ischemic limb and capillary density in the ischemic adductor muscle fibers compared with the control wild-type mice. The zinc-deficient mice also showed increased reactive oxygen species production after hindlimb ischemia. Nicotinamide adenine dinucleotide phosphate oxidase inhibitors ameliorated the zinc deficient-induced impairment of revascularization. The serum zinc levels were positively associated with the SPP in the CLTI patients. Multivariate regression analysis also revealed that the serum zinc levels were significantly correlated with the SPP in patients with CLTI.
Conclusions: Zinc deficiency impaired the rate of ischemia-induced revascularization through enhanced oxidative stress rates, suggesting that nutritional management for zinc sufficiency could be useful in CLTI prevention and treatment.
Keywords: Angiogenesis; Ischemia; Zinc.
© 2021 Published by Elsevier Inc. on behalf of the Society for Vascular Surgery.
Figures
Similar articles
-
Metformin stimulates ischemia-induced revascularization through an eNOS dependent pathway in the ischemic hindlimb mice model.J Vasc Surg. 2015 Feb;61(2):489-96. doi: 10.1016/j.jvs.2013.09.061. Epub 2014 Jul 1. J Vasc Surg. 2015. PMID: 24993950
-
Deletion of FHL2 gene impaired ischemia-induced blood flow recovery by modulating circulating proangiogenic cells.Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):709-17. doi: 10.1161/ATVBAHA.112.300318. Epub 2013 Feb 14. Arterioscler Thromb Vasc Biol. 2013. PMID: 23413425
-
Effect of revascularization on lower extremity muscle function in combined type 2 diabetes and critical limb threatening ischemia.Int Angiol. 2021 Aug;40(4):323-334. doi: 10.23736/S0392-9590.21.04661-7. Epub 2021 May 19. Int Angiol. 2021. PMID: 34008931
-
Diabetes Mellitus and Lower Extremity Peripheral Artery Disease.JMA J. 2021 Jul 15;4(3):225-231. doi: 10.31662/jmaj.2021-0042. Epub 2021 Jul 9. JMA J. 2021. PMID: 34414316 Free PMC article. Review.
-
Roles of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase in Angiogenesis: Isoform-Specific Effects.Antioxidants (Basel). 2017 Jun 3;6(2):40. doi: 10.3390/antiox6020040. Antioxidants (Basel). 2017. PMID: 28587189 Free PMC article. Review.
Cited by
-
Nutrition in the management of peripheral arterial disease: should we pay more attention to what our patients eat?JVS Vasc Sci. 2021 Dec 6;3:15-16. doi: 10.1016/j.jvssci.2021.11.003. eCollection 2022. JVS Vasc Sci. 2021. PMID: 35028600 Free PMC article. No abstract available.
-
Synthesis, Sensing Performance and DFT Studies of a Novel Coumarin-based Schiff Base As a Turn-on Fluorescence Probe for Zinc Ion Detection.J Fluoresc. 2025 Jan;35(1):307-315. doi: 10.1007/s10895-023-03510-x. Epub 2023 Dec 2. J Fluoresc. 2025. PMID: 38041792
-
Transition metals in angiogenesis - A narrative review.Mater Today Bio. 2023 Aug 3;22:100757. doi: 10.1016/j.mtbio.2023.100757. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37593220 Free PMC article. Review.
-
Strategic incorporation of metal ions in bone regenerative scaffolds: multifunctional platforms for advancing osteogenesis.Regen Biomater. 2025 Jul 2;12:rbaf068. doi: 10.1093/rb/rbaf068. eCollection 2025. Regen Biomater. 2025. PMID: 40755870 Free PMC article. Review.
References
-
- Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. - PubMed
-
- Strain J.J. Trace elements and cardiovascular disease. Bibl Nutr Dieta. 1998;54:127–140. - PubMed
-
- Alissa E.M., Bahijri S.M., Ferns G.A. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit. 2003;9:RA9–RA18. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

