Antibody profiles in COVID-19 convalescent plasma prepared with amotosalen/UVA pathogen reduction treatment
- PMID: 35128658
- PMCID: PMC9115453
- DOI: 10.1111/trf.16819
Antibody profiles in COVID-19 convalescent plasma prepared with amotosalen/UVA pathogen reduction treatment
Abstract
Background: COVID-19 convalescent plasma (CCP), from donors recovered from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, is one of the limited therapeutic options currently available for the treatment of critically ill patients with COVID-19. There is growing evidence that CCP may reduce viral loads and disease severity; and reduce mortality. However, concerns about the risk of transfusion-transmitted infections (TTI) and other complications associated with transfusion of plasma, remain. Amotosalen/UVA pathogen reduction treatment (A/UVA-PRT) of plasma offers a mitigation of TTI risk, and when combined with pooling has the potential to increase the diversity of the polyclonal SARS-CoV-2 neutralizing antibodies.
Study design and methods: This study assessed the impact of A/UVA-PRT on SARS-CoV-2 antibodies in 42 CCP using multiple complimentary assays including antigen binding, neutralizing, and epitope microarrays. Other mediators of CCP efficacy were also assessed.
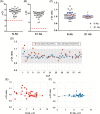
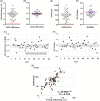
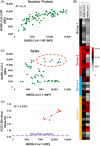
Results: A/UVA-PRT did not negatively impact antibodies to SARS-CoV-2 and other viral epitopes, had no impact on neutralizing activity or other potential mediators of CCP efficacy. Finally, immune cross-reactivity with other coronavirus antigens was observed raising the potential for neutralizing activity against other emergent coronaviruses.
Conclusion: The findings of this study support the selection of effective CCP combined with the use of A/UVA-PRT in the production of CCP for patients with COVID-19.
Keywords: FFP transfusion; plasma derivatives; transfusion-transmitted disease-other.
© 2022 The Authors. Transfusion published by Wiley Periodicals LLC on behalf of AABB.
Conflict of interest statement
AB, MVG, MG, and LMC are employees and shareholders of Cerus Corporation which markets the INTERCEPT Blood System. CTT, PVR, and DS are employees of ENable Biosciences which markets ADAP assay for SARS‐CoV‐2 detection. PLF is a shareholder in Antigen Discovery Inc. and Nano immune Inc. RRDA, GS, ZWM, MS, CDG, RM, OD, JR, DW, AZ, SK, and MPB have disclosed no conflicts of interest.
Figures



Comment in
-
Pathogen reduction technologies need to evaluate Fc-mediated antibody functions.Transfusion. 2022 Aug;62(8):1680-1681. doi: 10.1111/trf.16995. Transfusion. 2022. PMID: 35932391 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

