Prasugrel-based De-Escalation of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With STEMI
- PMID: 35129316
- PMCID: PMC8989793
- DOI: 10.4070/kcj.2021.0293
Prasugrel-based De-Escalation of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With STEMI
Erratum in
-
Erratum: Correction of Text in the Article "Prasugrel-based De-Escalation of Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in Patients With STEMI".Korean Circ J. 2022 Jun;52(6):483-484. doi: 10.4070/kcj.2022.0999. Korean Circ J. 2022. PMID: 35656907 Free PMC article.
Abstract
Background and objectives: De-escalation of dual-antiplatelet therapy through dose reduction of prasugrel improved net adverse clinical events (NACEs) after acute coronary syndrome (ACS), mainly through the reduction of bleeding without an increase in ischemic outcomes. Whether the benefits of de-escalation are sustained in highly thrombotic conditions such as ST-elevation myocardial infarction (STEMI) is unknown. We aimed to assess the efficacy and safety of de-escalation therapy in patients with STEMI or non-ST-segment elevation ACS (NSTE-ACS).
Methods: This is a pre-specified subgroup analysis of the HOST-REDUCE-POLYTECH-ACS trial. ACS patients were randomized to prasugrel de-escalation (5 mg daily) or conventional dose (10 mg daily) at 1-month post-percutaneous coronary intervention. The primary endpoint was a NACE, defined as a composite of all-cause death, non-fatal myocardial infarction, stent thrombosis, clinically driven revascularization, stroke, and bleeding events of grade ≥2 Bleeding Academic Research Consortium (BARC) criteria at 1 year.
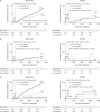
Results: Among 2,338 patients included in the randomization, 326 patients were diagnosed with STEMI. In patients with NSTE-ACS, the risk of the primary endpoint was significantly reduced with de-escalation (hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.48-0.89; p=0.006 for de-escalation vs. conventional), mainly driven by a reduced bleeding. However, in those with STEMI, there was no difference in the occurrence of the primary outcome (HR, 1.04; 95% CI, 0.48-2.26; p=0.915; p for interaction=0.271).
Conclusions: Prasugrel dose de-escalation reduced the rate of NACE and bleeding, without increasing the rate of ischemic events in NSTE-ACS patients but not in STEMI patients.
Keywords: Acute coronary syndrome; Non-ST elevated myocardial infarction; Percutaneous coronary intervention; Prasugrel; ST elevation myocardial infarction.
Copyright © 2022. The Korean Society of Cardiology.
Conflict of interest statement
Dr. Hyo-Soo Kim has received research grants or speaker's fees from Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, Dio, Medtronic, Abbott Vascular, Edwards Life Science, Amgen, and Behringer Ingelheim, outside of the submitted work. Dr. Kyung Woo Park reports speaker's fees from Daiichi Sankyo, AstraZeneca, Sanofi, Bristol-Myers Squibb, Bayer, and Pfizer, outside of the submitted work. All other authors declare no competing interests.
Figures


References
-
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. - PubMed
-
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. - PubMed
-
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. - PubMed
-
- Kang J, Park KW, Ki YJ, et al. Development and validation of an ischemic and bleeding risk evaluation tool in East Asian patients receiving percutaneous coronary intervention. Thromb Haemost. 2019;119:1182–1193. - PubMed
-
- Kang J, Park KW, Palmerini T, et al. Racial differences in ischaemia/bleeding risk trade-off during anti-platelet therapy: individual patient level landmark meta-analysis from seven RCTs. Thromb Haemost. 2019;119:149–162. - PubMed
LinkOut - more resources
Full Text Sources

