Endothelial cells: potential novel regulators of renal inflammation
- PMID: 35129369
- PMCID: PMC8897017
- DOI: 10.1152/ajprenal.00371.2021
Endothelial cells: potential novel regulators of renal inflammation
Abstract
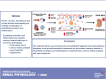
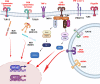
Substantial evidence has supported the role of endothelial cell (EC) activation and dysfunction in the development of hypertension, chronic kidney disease (CKD), and lupus nephritis (LN). In both humans and experimental models of hypertension, CKD, and LN, ECs become activated and release potent mediators of inflammation including cytokines, chemokines, and reactive oxygen species that cause EC dysfunction, tissue damage, and fibrosis. Factors that activate the endothelium include inflammatory cytokines, mechanical stretch, and pathological shear stress. These signals can activate the endothelium to promote upregulation of adhesion molecules, such as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1, which promote leukocyte adhesion and migration to the activated endothelium. More importantly, it is now recognized that some of these signals may in turn promote endothelial antigen presentation through major histocompatibility complex II. In this review, we will consider in-depth mechanisms of endothelial activation and the novel mechanism of endothelial antigen presentation. Moreover, we will discuss these proinflammatory events in renal pathologies and consider possible new therapeutic approaches to limit the untoward effects of endothelial inflammation in hypertension, CKD, and LN.
Keywords: antigen presentation; endothelial cells; inflammation; kidney injury; renal.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Proinflammatory Signature of the Dysfunctional Endothelium in Pulmonary Hypertension. Role of the Macrophage Migration Inhibitory Factor/CD74 Complex.Am J Respir Crit Care Med. 2015 Oct 15;192(8):983-97. doi: 10.1164/rccm.201402-0322OC. Am J Respir Crit Care Med. 2015. PMID: 26203495
-
Interleukin-1α Is a Central Regulator of Leukocyte-Endothelial Adhesion in Myocardial Infarction and in Chronic Kidney Disease.Circulation. 2021 Sep 14;144(11):893-908. doi: 10.1161/CIRCULATIONAHA.121.053547. Epub 2021 Jul 1. Circulation. 2021. PMID: 34192892
-
Estradiol Inhibits Cytokine-Induced Expression of VCAM-1 and ICAM-1 in Cultured Human Endothelial Cells Via AMPK/PPARα Activation.Cell Biochem Biophys. 2015 Jul;72(3):709-17. doi: 10.1007/s12013-015-0522-y. Cell Biochem Biophys. 2015. Retraction in: Cell Biochem Biophys. 2023 Mar;81(1):165. doi: 10.1007/s12013-022-01111-2. PMID: 25627546 Retracted.
-
Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants.Antioxid Redox Signal. 2011 Sep 15;15(6):1607-38. doi: 10.1089/ars.2010.3522. Epub 2011 May 11. Antioxid Redox Signal. 2011. PMID: 21050132 Free PMC article. Review.
-
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
Cited by
-
An Overview of Chronic Kidney Disease Pathophysiology: The Impact of Gut Dysbiosis and Oral Disease.Biomedicines. 2023 Nov 12;11(11):3033. doi: 10.3390/biomedicines11113033. Biomedicines. 2023. PMID: 38002033 Free PMC article. Review.
-
Long-Term Effects of Suramin on Renal Function in Streptozotocin-Induced Diabetes in Rats.Int J Mol Sci. 2023 Sep 28;24(19):14671. doi: 10.3390/ijms241914671. Int J Mol Sci. 2023. PMID: 37834118 Free PMC article.
-
Recent advances on immunity and hypertension: the new cells on the kidney block.Am J Physiol Renal Physiol. 2025 Mar 1;328(3):F301-F315. doi: 10.1152/ajprenal.00309.2024. Epub 2025 Jan 24. Am J Physiol Renal Physiol. 2025. PMID: 39853324 Free PMC article. Review.
-
Localization of natriuretic peptide receptors A, B, and C in healthy and diseased mouse kidneys.Pflugers Arch. 2023 Mar;475(3):343-360. doi: 10.1007/s00424-022-02774-9. Epub 2022 Dec 8. Pflugers Arch. 2023. PMID: 36480070 Free PMC article.
-
Leukocyte-endothelial interaction in CKD.Clin Kidney J. 2023 Jun 8;16(11):1845-1860. doi: 10.1093/ckj/sfad135. eCollection 2023 Nov. Clin Kidney J. 2023. PMID: 37915921 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

