International Center-Level Variation in Utilization of Completion Lymph Node Dissection and Adjuvant Systemic Therapy for Sentinel Lymph Node-Positive Melanoma at Major Referral Centers
- PMID: 35129464
- PMCID: PMC10097464
- DOI: 10.1097/SLA.0000000000005370
International Center-Level Variation in Utilization of Completion Lymph Node Dissection and Adjuvant Systemic Therapy for Sentinel Lymph Node-Positive Melanoma at Major Referral Centers
Abstract
Objective: The aim of this study was to determine overall trends and center-level variation in utilization of completion lymph node dissection (CLND) and adjuvant systemic therapy for sentinel lymph node (SLN)-positive melanoma.
Summary background data: Based on recent clinical trials, management options for SLN-positive melanoma now include effective adjuvant systemic therapy and nodal observation instead of CLND. It is unknown how these findings have shaped practice or how these contemporaneous developments have influenced their respective utilization.
Methods: We performed an international cohort study at 21 melanoma referral centers in Australia, Europe, and the United States that treated adults with SLN-positive melanoma and negative distant staging from July 2017 to June 2019. We used generalized linear and multinomial logistic regression models with random intercepts for each center to assess center-level variation in CLND and adjuvant systemic treatment, adjusting for patient and disease-specific characteristics.
Results: Among 1109 patients, performance of CLND decreased from 28% to 8% and adjuvant systemic therapy use increased from 29 to 60%. For both CLND and adjuvant systemic treatment, the most influential factors were nodal tumor size, stage, and location of treating center. There was notable variation among treating centers in management of stage IIIA patients and use of CLND with adjuvant systemic therapy versus nodal observation alone for similar risk patients.
Conclusions: There has been an overall decline in CLND and simultaneous adoption of adjuvant systemic therapy for patients with SLN-positive melanoma though wide variation in practice remains. Accounting for differences in patient mix, location of care contributed significantly to the observed variation.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

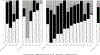
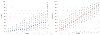

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

