Pseudomonas Aeruginosa Theft Biofilm Require Host Lipids of Cutaneous Wound
- PMID: 35129518
- PMCID: PMC9275790
- DOI: 10.1097/SLA.0000000000005252
Pseudomonas Aeruginosa Theft Biofilm Require Host Lipids of Cutaneous Wound
Abstract
Objective: This work addressing complexities in wound infection, seeks to test the reliance of bacterial pathogen Pseudomonas aeruginosa (PA) on host skin lipids to form biofilm with pathological consequences.
Background: PA biofilm causes wound chronicity. Both CDC as well as NIH recognizes biofilm infection as a threat leading to wound chronicity. Chronic wounds on lower extremities often lead to surgical limb amputation.
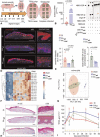
Methods: An established preclinical porcine chronic wound biofilm model, infected with PA or Pseudomonas aeruginosa ceramidase mutant (PA ∆Cer ), was used.
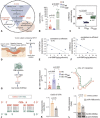
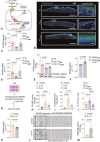
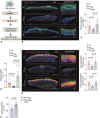
Results: We observed that bacteria drew resource from host lipids to induce PA ceramidase expression by three orders of magnitude. PA utilized product of host ceramide catabolism to augment transcription of PA ceramidase. Biofilm formation was more robust in PA compared to PA ∆Cer . Downstream products of such metabolism such as sphingosine and sphingosine-1-phosphate were both directly implicated in the induction of ceramidase and inhibition of peroxisome proliferator-activated receptor (PPAR)δ, respectively. PA biofilm, in a ceram-idastin-sensitive manner, also silenced PPARδ via induction of miR-106b. Low PPARδ limited ABCA12 expression resulting in disruption of skin lipid homeostasis. Barrier function of the wound-site was thus compromised.
Conclusions: This work demonstrates that microbial pathogens must co-opt host skin lipids to unleash biofilm pathogenicity. Anti-biofilm strategies must not necessarily always target the microbe and targeting host lipids at risk of infection could be productive. This work may be viewed as a first step, laying fundamental mechanistic groundwork, toward a paradigm change in biofilm management.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- Wolcott R, Dowd S. The role of biofilms: are we hitting the right target? Plast Reconstr Surg. 2011;127(suppl 1):28S–35S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

