Consensus Recommendation for Mouse Models of Ocular Hypertension to Study Aqueous Humor Outflow and Its Mechanisms
- PMID: 35129590
- PMCID: PMC8842499
- DOI: 10.1167/iovs.63.2.12
Consensus Recommendation for Mouse Models of Ocular Hypertension to Study Aqueous Humor Outflow and Its Mechanisms
Abstract
Due to their similarities in anatomy, physiology, and pharmacology to humans, mice are a valuable model system to study the generation and mechanisms modulating conventional outflow resistance and thus intraocular pressure. In addition, mouse models are critical for understanding the complex nature of conventional outflow homeostasis and dysfunction that results in ocular hypertension. In this review, we describe a set of minimum acceptable standards for developing, characterizing, and utilizing mouse models of open-angle ocular hypertension. We expect that this set of standard practices will increase scientific rigor when using mouse models and will better enable researchers to replicate and build upon previous findings.
Conflict of interest statement
Disclosure:
Figures
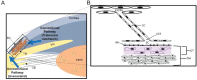
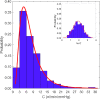
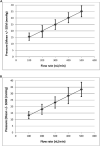

References
-
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998; 126: 498–505. - PubMed
-
- The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000; 130: 429–440. - PubMed
-
- Gordon MO, Beiser JA, Brandt JD, et al. .. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 714–720; discussion 829–830. - PubMed
-
- Higginbotham EJ, Gordon MO, Beiser JA, et al. .. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004; 122: 813–820. - PubMed
-
- Leske MC, Heijl A, Hyman L, Bengtsson B.. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999; 106: 2144–2153. - PubMed
Publication types
MeSH terms
Grants and funding
- I01 RX001163/RX/RRD VA/United States
- R01 EY026885/EY/NEI NIH HHS/United States
- R01 EY019643/EY/NEI NIH HHS/United States
- R01 EY031817/EY/NEI NIH HHS/United States
- R01 EY013178/EY/NEI NIH HHS/United States
- R01 EY025721/EY/NEI NIH HHS/United States
- R01 EY026177/EY/NEI NIH HHS/United States
- R01 EY027733/EY/NEI NIH HHS/United States
- P30 EY031631/EY/NEI NIH HHS/United States
- R01 EY026529/EY/NEI NIH HHS/United States
- R01 EY032905/EY/NEI NIH HHS/United States
- R21 EY028671/EY/NEI NIH HHS/United States
- P30 EY005722/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- R01 EY026048/EY/NEI NIH HHS/United States
- K08 EY032202/EY/NEI NIH HHS/United States
- R01 EY026962/EY/NEI NIH HHS/United States
- R21 EY033073/EY/NEI NIH HHS/United States
- R01 EY017006/EY/NEI NIH HHS/United States
- R01 EY030238/EY/NEI NIH HHS/United States
- R01 EY031700/EY/NEI NIH HHS/United States
- R01 EY028616/EY/NEI NIH HHS/United States
- R01 EY021727/EY/NEI NIH HHS/United States
- R01 EY021205/EY/NEI NIH HHS/United States
- R01 EY027920/EY/NEI NIH HHS/United States
- R01 EY029320/EY/NEI NIH HHS/United States
- K99 EY032547/EY/NEI NIH HHS/United States
- R01 EY034534/EY/NEI NIH HHS/United States
- R01 EY025543/EY/NEI NIH HHS/United States
- P30 EY014800/EY/NEI NIH HHS/United States
- R01 EY018590/EY/NEI NIH HHS/United States
- I01 RX002860/RX/RRD VA/United States
- R01 EY022634/EY/NEI NIH HHS/United States

