Bioavailability and Safety of a New Highly Concentrated Midazolam Nasal Spray Compared to Buccal and Intravenous Midazolam Treatment in Chinese Healthy Volunteers
- PMID: 35129802
- PMCID: PMC9095771
- DOI: 10.1007/s40120-022-00329-9
Bioavailability and Safety of a New Highly Concentrated Midazolam Nasal Spray Compared to Buccal and Intravenous Midazolam Treatment in Chinese Healthy Volunteers
Abstract
Introduction: Buccal midazolam treatment is licensed in the European Union for prolonged acute convulsive seizures in children and adolescents, but the buccal pathway is often hampered by jaw clenching, hypersalivation, or uncontrolled swallowing. Midazolam formulations that are more secure, reliable, and faster for use are needed in the acute setting. Pharmacokinetics and comparative bioavailability of intranasally administered midazolam and two midazolam intravenous solutions administered buccally or intravenously in healthy adults were evaluated.
Methods: In this phase 1, open-label, randomized, single-dose, three-period, three-sequence crossover study, 12 healthy adults (19-41 years) were randomly assigned to receive 2.5 mg midazolam intranasally; 2.5 mg midazolam intravenously; 2.5 mg midazolam buccally. Blood samples were collected for 10 h post dose to determine pharmacokinetic profiles. Adverse events and vital signs were recorded.
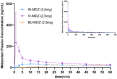
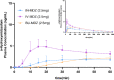
Results: Intranasal administration of 2.5 mg midazolam demonstrated a more rapid median time to Cmax compared to buccal administration of midazolam (Tmax, 12.6 min vs. 45 min; Cmax, 38.33 ng/ml vs. 24.97 ng/ml). The antiepileptic effect of intranasal and buccal midazolam treatment lasted less than 4 h and generally did not differ from intravenously administered midazolam. No serious adverse events or deaths were reported, and no treatment-emergent adverse events led to study discontinuation.
Conclusion: Intranasal administration of midazolam may be a preferable alternative to the currently approve buccal midazolam treatment for prolonged acute convulsive seizures in children and adolescents.
Trial registration: This study is registered at the Chinese Clinical Trial [ http://www.chictr.org.cn ] (ChiCTR2000032595) on 3 May, 2020.
Keywords: Bioavailability; Buccal midazolam; Intravenous midazolam; Midazolam; Nasal spray; Safety.
© 2022. The Author(s).
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

