Orientation of Cell Polarity by Chemical Gradients
- PMID: 35130037
- PMCID: PMC9549416
- DOI: 10.1146/annurev-biophys-110821-071250
Orientation of Cell Polarity by Chemical Gradients
Abstract
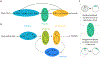
Accurate decoding of spatial chemical landscapes is critical for many cell functions. Eukaryotic cells decode local chemical gradients to orient growth or movement in productive directions. Recent work on yeast model systems, whose gradient sensing pathways display much less complexity than those in animal cells, has suggested new paradigms for how these very small cells successfully exploit information in noisy and dynamic pheromone gradients to identify their mates. Pheromone receptors regulate a polarity circuit centered on the conserved Rho-family GTPase, Cdc42. The polarity circuit contains both positive and negative feedback pathways, allowing spontaneous symmetry breaking and also polarity site disassembly and relocation. Cdc42 orients the actin cytoskeleton, leading to focused vesicle traffic that promotes movement of the polarity site and also reshapes the cortical distribution of receptors at the cell surface. In this article, we review the advances from work on yeasts and compare them with the excitable signaling pathways that have been revealed in chemotactic animal cells.
Keywords: Cdc42; chemotaxis; chemotropism.
Figures

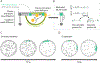

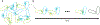

Similar articles
-
Chemotactic movement of a polarity site enables yeast cells to find their mates.Proc Natl Acad Sci U S A. 2021 Jun 1;118(22):e2025445118. doi: 10.1073/pnas.2025445118. Proc Natl Acad Sci U S A. 2021. PMID: 34050026 Free PMC article.
-
Mechanistic insights into actin-driven polarity site movement in yeast.Mol Biol Cell. 2020 May 1;31(10):1085-1102. doi: 10.1091/mbc.E20-01-0040. Epub 2020 Mar 18. Mol Biol Cell. 2020. PMID: 32186970 Free PMC article.
-
A Cellular System for Spatial Signal Decoding in Chemical Gradients.Dev Cell. 2015 Nov 23;35(4):458-70. doi: 10.1016/j.devcel.2015.10.013. Epub 2015 Nov 12. Dev Cell. 2015. PMID: 26585298
-
Symmetry breaking in the life cycle of the budding yeast.Cold Spring Harb Perspect Biol. 2009 Sep;1(3):a003384. doi: 10.1101/cshperspect.a003384. Cold Spring Harb Perspect Biol. 2009. PMID: 20066112 Free PMC article. Review.
-
Spontaneous cell polarization: Feedback control of Cdc42 GTPase breaks cellular symmetry.Bioessays. 2015 Nov;37(11):1193-201. doi: 10.1002/bies.201500077. Epub 2015 Sep 4. Bioessays. 2015. PMID: 26338468 Review.
Cited by
-
Negative feedback equalizes polarity sites in a multi-budding yeast.Curr Biol. 2025 Jul 7;35(13):3022-3034.e4. doi: 10.1016/j.cub.2025.05.011. Epub 2025 Jun 6. Curr Biol. 2025. PMID: 40482649
-
Intracellular Ca2+ waves in mammalian cells.Biol Futur. 2025 Sep;76(3):293-313. doi: 10.1007/s42977-025-00270-6. Epub 2025 Jun 29. Biol Futur. 2025. PMID: 40581908 Review.
-
Measuring Piezo1 and Actin Polarity in Chemokine-Stimulated Jurkat Cells During Live-Cell Imaging.Bio Protoc. 2024 Oct 5;14(19):e5079. doi: 10.21769/BioProtoc.5079. eCollection 2024 Oct 5. Bio Protoc. 2024. PMID: 39399591 Free PMC article.
-
PIP5K-Ras bistability initiates plasma membrane symmetry breaking to regulate cell polarity and migration.bioRxiv [Preprint]. 2024 Sep 15:2024.09.15.613115. doi: 10.1101/2024.09.15.613115. bioRxiv. 2024. PMID: 39314378 Free PMC article. Preprint.
-
Durotaxis and negative durotaxis: where should cells go?Commun Biol. 2023 Nov 16;6(1):1169. doi: 10.1038/s42003-023-05554-y. Commun Biol. 2023. PMID: 37973823 Free PMC article. Review.
References
-
- Abdul-Ganiyu R, Venegas LA, Wang X, Puerner C, Arkowitz RA, et al. 2021. Phosphorylated Gβ is a directional cue during yeast gradient tracking. Sci Signal. 14(682):eabf4710. - PubMed
-
- Andrew N, Insall RH. 2007. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 9(2):193–200 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

