CRC-derived exosomes containing the RNA binding protein HuR promote lung cell proliferation by stabilizing c-Myc mRNA
- PMID: 35130122
- PMCID: PMC8824215
- DOI: 10.1080/15384047.2022.2034455
CRC-derived exosomes containing the RNA binding protein HuR promote lung cell proliferation by stabilizing c-Myc mRNA
Abstract
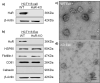
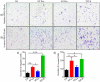
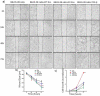
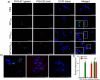
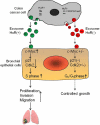
HuR overexpression is related to poor survival in patients with colon cancer. HuR overexpression leads to stabilization of tumor-promoting mRNAs by binding to 3'UTR-resident AREs. Exosomes, nanosized lipid bilayer vesicles, mediate many steps in cancer progression. The potential role of exosomal HuR in colon cancer lung metastasis is unclear. HuR expression was assessed immunohistochemically in tumor tissue samples from 20 patients with metastatic or nonmetastatic colon cancer and colon cancer lung metastasis and benign lung disease samples from ten patients. Exosomes were isolated from HCT116 WT and HuR KO colon cancer cells, and uptake of PKH67- and PKH26-labeled exosomes by BEAS-2B cells was evaluated using fluorescence and confocal microscopy. C-Myc and p21protein and mRNA levels were measured by western blotting and RT-qPCR, respectively. In clinical patients, HuR overexpression was significantly enhanced in colon tissues of patients with lung metastasis. HuR expression was higher in lung tissue with metastasis of colonic origin than with benign lung disease. The effect of HuR-containing CRC exosomes compared to HuR-deficient exosomes on wound closure was observed as enhanced proliferation. BEAS-2B cell migration and invasion were enhanced after HuR-containing exosomes treatment. BEAS-2B cells showed similar uptake of PKH67 (HCT116 WT)- and PKH26 (HCT116 HuR KO)-labeled exosomes. Exosomal HuR stabilized c-Myc mRNA and downregulated p21 expression, leading to G1/S transition, in human bronchial epithelial cells. HuR overexpression is associated with lung metastasis in colon cancer patients. Exosomal HuR derived from colon cancer cells alter the biological effect on normal lung epithelial cells.
Keywords: colon cancer; exosomes; extracellular vesicle; human antigen R; proliferation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Circular RNA circRHOBTB3 represses metastasis by regulating the HuR-mediated mRNA stability of PTBP1 in colorectal cancer.Theranostics. 2021 Jun 1;11(15):7507-7526. doi: 10.7150/thno.59546. eCollection 2021. Theranostics. 2021. PMID: 34158864 Free PMC article.
-
Exosomal microRNAs derived from colon cancer cells promote tumor progression by suppressing fibroblast TP53 expression.Cancer Sci. 2019 Aug;110(8):2396-2407. doi: 10.1111/cas.14084. Epub 2019 Jul 9. Cancer Sci. 2019. PMID: 31148360 Free PMC article.
-
MiR-155-5p controls colon cancer cell migration via post-transcriptional regulation of Human Antigen R (HuR).Cancer Lett. 2018 May 1;421:145-151. doi: 10.1016/j.canlet.2018.02.026. Epub 2018 Feb 20. Cancer Lett. 2018. PMID: 29471005
-
HuR as a molecular target for cancer therapeutics and immune-related disorders.Adv Drug Deliv Rev. 2022 Sep;188:114442. doi: 10.1016/j.addr.2022.114442. Epub 2022 Jul 8. Adv Drug Deliv Rev. 2022. PMID: 35817212 Free PMC article. Review.
-
The RNA-binding protein HuR in human cancer: A friend or foe?Adv Drug Deliv Rev. 2022 May;184:114179. doi: 10.1016/j.addr.2022.114179. Epub 2022 Mar 3. Adv Drug Deliv Rev. 2022. PMID: 35248670 Free PMC article. Review.
Cited by
-
Extracellular transfer of HuR promotes acquired cisplatin resistance in esophageal cancer cells.Cancer Biol Ther. 2025 Dec;26(1):2495999. doi: 10.1080/15384047.2025.2495999. Epub 2025 Apr 23. Cancer Biol Ther. 2025. PMID: 40269355 Free PMC article.
-
Extracellular Vesicles in Colorectal Cancer: From Tumor Growth and Metastasis to Biomarkers and Nanomedications.Cancers (Basel). 2023 Feb 9;15(4):1107. doi: 10.3390/cancers15041107. Cancers (Basel). 2023. PMID: 36831450 Free PMC article. Review.
-
The multifaceted role of extracellular vesicles (EVs) in colorectal cancer: metastasis, immune suppression, therapy resistance, and autophagy crosstalk.J Transl Med. 2024 May 13;22(1):452. doi: 10.1186/s12967-024-05267-8. J Transl Med. 2024. PMID: 38741166 Free PMC article. Review.
-
CircREOS suppresses lipid synthesis and osteosarcoma progression through inhibiting HuR-mediated MYC activation.J Cancer. 2023 Apr 2;14(6):916-926. doi: 10.7150/jca.83106. eCollection 2023. J Cancer. 2023. PMID: 37151387 Free PMC article.
-
Gut commensal Bifidobacterium-derived extracellular vesicles modulate the therapeutic effects of anti-PD-1 in lung cancer.Nat Commun. 2025 Apr 12;16(1):3500. doi: 10.1038/s41467-025-58553-4. Nat Commun. 2025. PMID: 40221398 Free PMC article.
References
-
- Usui G, Masuda Y, Hashimoto H, Kusakabe M, Nakajima K, Tsunoda H, Matsuhashi N, Harihara Y, Horiuchi H, Morikawa T. Colon metastasis from microscopic serous carcinoma of the fallopian tube fimbria mimicking a primary colon cancer. Int J Surg Pathol. 2019;27(4):390–395. PMID: 30663467. doi:10.1177/1066896918824028 - DOI - PubMed
-
- Rosenberger L, Ezquer M, Lillo-Vera F, Pedraza PL, Ortuzar MI, Gonzalez PL, Figueroa-Valdes AI, Cuenca J, Ezquer F, Khoury M, et al. Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci Rep. 2019;9(1):663. PMID: 30679544. doi:10.1038/s41598-018-36855-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
