Bacterial MerR family transcription regulators: activationby distortion
- PMID: 35130613
- PMCID: PMC9909328
- DOI: 10.3724/abbs.2021003
Bacterial MerR family transcription regulators: activationby distortion
Abstract
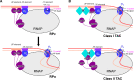
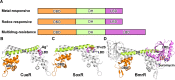
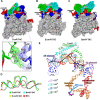
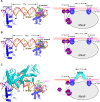
Transcription factors (TFs) modulate gene expression by regulating the accessibility of promoter DNA to RNA polymerases (RNAPs) in bacteria. The MerR family TFs are a large class of bacterial proteins unique in their physiological functions and molecular action: they function as transcription repressors under normal circumstances, but rapidly transform to transcription activators under various cellular triggers, including oxidative stress, imbalance of cellular metal ions, and antibiotic challenge. The promoters regulated by MerR TFs typically contain an abnormal long spacer between the -35 and -10 elements, where MerR TFs bind and regulate transcription activity through unique mechanisms. In this review, we summarize the function, ligand reception, DNA recognition, and molecular mechanism of transcription regulation of MerR-family TFs.
Keywords: MerR; RNA polymerase; gene expression; gene transcription; transcription factor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Chamberlin M, Berg P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli . Proc Natl Acad Sci U S A. . 1962;48:81–94. doi: 10.1073/pnas.48.1.81. - DOI - PMC - PubMed
-
- Feklistov A, Sharon BD, Darst SA, Gross CA. Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol. . 2014;68:357–376. doi: 10.1146/annurev-micro-092412-155737. - DOI - PubMed
-
- Seshasayee AS, Sivaraman K, Luscombe NM. An overview of prokaryotic transcription factors: a summary of function and occurrence in bacterial genomes. Subcell Biochem. 2011,52: 7-23 - PubMed
-
- Browning DF, Busby SJW. Local and global regulation of transcription initiation in bacteria. Nat Rev Microbiol. . 2016;14:638–650. doi: 10.1038/nrmicro.2016.103. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

