The residues 4 to 6 at the N-terminus in particular modulate fibril propagation of β-microglobulin
- PMID: 35130623
- PMCID: PMC9909321
- DOI: 10.3724/abbs.2021017
The residues 4 to 6 at the N-terminus in particular modulate fibril propagation of β-microglobulin
Abstract
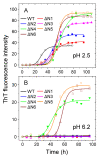
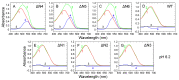
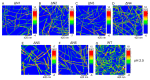
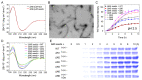
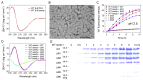
The ΔN6 truncation is the main posttranslational modification of β-microglobulin (βM) found in dialysis-related amyloid. Investigation of the interaction of wild-type (WT) βM with N-terminally truncated variants is therefore of medical relevance. However, it is unclear which residues among the six residues at the N-terminus are crucial to the interactions and the modulation of amyloid fibril propagation of βM. We herein analyzed homo- and heterotypic seeding of amyloid fibrils of WT human βM and its N-terminally-truncated variants ΔN1 to ΔN6, lacking up to six residues at the N-terminus. At acidic pH 2.5, we produced amyloid fibrils from recombinant, WT βM and its six truncated variants, and found that ΔN6 βM fibrils exhibit a significantly lower conformational stability than WT βM fibrils. Importantly, under more physiological conditions (pH 6.2), we assembled amyloid fibrils only from recombinant, ΔN4, ΔN5, and ΔN6 βM but not from WT βM and its three truncated variants ΔN1 to ΔN3. Notably, the removal of the six, five or four residues at the N-terminus leads to enhanced fibril formation, and homo- and heterotypic seeding of ΔN6 fibrils strongly promotes amyloid fibril formation of WT βM and its six truncated variants, including at more physiological pH 6.2. Collectively, these results demonstrated that the residues 4 to 6 at the N-terminus particularly modulate amyloid fibril propagation of βM and the interactions of WT βM with N-terminally truncated variants, potentially indicating the direct relevance to the involvement of the protein's aggregation in dialysis-related amyloidosis.
Keywords: amyloid fibril; atomic force microscopy; conformational stability; fibril propagation; human β-microglobulin; truncated variant.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Secondary structure in the core of amyloid fibrils formed from human β₂m and its truncated variant ΔN6.J Am Chem Soc. 2014 Apr 30;136(17):6313-25. doi: 10.1021/ja4126092. Epub 2014 Apr 16. J Am Chem Soc. 2014. PMID: 24679070 Free PMC article.
-
Extracellular matrix components modulate different stages in β2-microglobulin amyloid formation.J Biol Chem. 2019 Jun 14;294(24):9392-9401. doi: 10.1074/jbc.RA119.008300. Epub 2019 Apr 17. J Biol Chem. 2019. PMID: 30996004 Free PMC article.
-
Macromolecular crowding favors the fibrillization of β2-microglobulin by accelerating the nucleation step and inhibiting fibril disassembly.Biochim Biophys Acta. 2016 Nov;1864(11):1609-19. doi: 10.1016/j.bbapap.2016.07.012. Epub 2016 Jul 30. Biochim Biophys Acta. 2016. PMID: 27481166
-
Structural stability of amyloid fibrils of beta(2)-microglobulin in comparison with its native fold.Biochim Biophys Acta. 2005 Nov 10;1753(1):64-75. doi: 10.1016/j.bbapap.2005.08.002. Epub 2005 Aug 24. Biochim Biophys Acta. 2005. PMID: 16213801 Review.
-
Limited proteolysis in the investigation of beta2-microglobulin amyloidogenic and fibrillar states.Biochim Biophys Acta. 2005 Nov 10;1753(1):44-50. doi: 10.1016/j.bbapap.2005.09.004. Epub 2005 Sep 23. Biochim Biophys Acta. 2005. PMID: 16213198 Review.
References
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. . 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. - DOI - PubMed
-
- Owen MC, Gnutt D, Gao M, Wärmländer SK, Jarvet J, Gräslund A, Winter R, et al. Effects of in vivo conditions on amyloid aggregation . Chem Soc Rev. . 2019;48:3946–3996. doi: 10.1039/C8CS00034D. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

