Reversing chemorefraction in colorectal cancer cells by controlling mucin secretion
- PMID: 35131032
- PMCID: PMC8846583
- DOI: 10.7554/eLife.73926
Reversing chemorefraction in colorectal cancer cells by controlling mucin secretion
Abstract
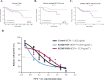
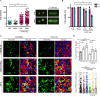
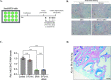
Fifteen percent of colorectal cancer (CRC) cells exhibit a mucin hypersecretory phenotype, which is suggested to provide resistance to immune surveillance and chemotherapy. We now formally show that CRC cells build a barrier to chemotherapeutics by increasing mucins' secretion. We show that low levels of KChIP3, a negative regulator of mucin secretion (Cantero-Recasens et al., 2018), is a risk factor for CRC patients' relapse in a subset of untreated tumours. Our results also reveal that cells depleted of KChIP3 are four times more resistant (measured as cell viability and DNA damage) to chemotherapeutics 5-fluorouracil + irinotecan (5-FU+iri.) compared to control cells, whereas KChIP3-overexpressing cells are 10 times more sensitive to killing by chemotherapeutics. A similar increase in tumour cell death is observed upon chemical inhibition of mucin secretion by the sodium/calcium exchanger (NCX) blockers (Mitrovic et al., 2013). Finally, sensitivity of CRC patient-derived organoids to 5-FU+iri. increases 40-fold upon mucin secretion inhibition. Reducing mucin secretion thus provides a means to control chemoresistance of mucinous CRC cells and other mucinous tumours.
Keywords: 5-FU+iri.; KChIP3; cell biology; chemoresistance; chemotherapy; colorectal cancer; human; mucins.
© 2022, Cantero-Recasens et al.
Conflict of interest statement
GC, JA, TL, MG, MI, LE No competing interests declared, VM Senior editor, eLife
Figures




References
-
- Cantero-Recasens G, Butnaru CM, Brouwers N, Mitrovic S, Valverde MA, Malhotra V. Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca2+-induced mucin secretion from goblet cells. The Journal of Biological Chemistry. 2019;294:816–826. doi: 10.1074/jbc.RA117.000848. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

