Cholinergic system changes in Parkinson's disease: emerging therapeutic approaches
- PMID: 35131038
- PMCID: PMC8985079
- DOI: 10.1016/S1474-4422(21)00377-X
Cholinergic system changes in Parkinson's disease: emerging therapeutic approaches
Abstract
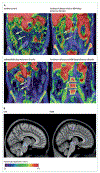
In patients with Parkinson's disease, heterogeneous cholinergic system changes can occur in different brain regions. These changes correlate with a range of clinical features, both motor and non-motor, that are refractory to dopaminergic therapy, and can be conceptualised within a systems-level framework in which nodal deficits can produce circuit dysfunctions. The topographies of cholinergic changes overlap with neural circuitries involved in sleep and cognitive, motor, visuo-auditory perceptual, and autonomic functions. Cholinergic deficits within cognition network hubs predict cognitive deficits better than do total brain cholinergic changes. Postural instability and gait difficulties are associated with cholinergic system changes in thalamic, caudate, limbic, neocortical, and cerebellar nodes. Cholinergic system deficits can involve also peripheral organs. Hypercholinergic activity of mesopontine cholinergic neurons in people with isolated rapid eye movement (REM) sleep behaviour disorder, as well as in the hippocampi of cognitively normal patients with Parkinson's disease, suggests early compensation during the prodromal and early stages of Parkinson's disease. Novel pharmacological and neurostimulation approaches could target the cholinergic system to treat motor and non-motor features of Parkinson's disease.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NIB has received research funding from the US National Institutes of Health (NIH), Department of Veterans Affairs, Parkinson's Foundation, Farmer Family Foundation Parkinson's Research Initiative, Michael J Fox Foundation, Eisai, and EIP Pharma. NIB has participated in an Eisai advisory board and received in-kind research support from Expansion Therapeutics and Innovative Health Solutions. AJY is supported by the UK National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre and has received funding from Parkinson's UK, Dunhill Medical Trust, NIHR, Weston Brain Institute, Lewy Body Society, Cure Parkinson's Trust, the Michael J Fox Foundation, and Intercept Pharmaceuticals. AJY has received in-kind research support (equipment) from electroCore. RSW has received funding from the Wellcome Trust, University College London Hospital Biomedical Research Centre, and the British Medical Association, speaker fees from GE Healthcare, and writing fees from Britannia. EM has received an educational grant from Boston Scientific and honoraria from Medtronic and Newronika. MSM has received research funding from the NIH, Harry T Mangurian Foundation, and Tyler's Hope for Dystonia. PB has received research funding from Danish Parkinson's Disease Association and the Lundbeck Foundation. MAB has received research funding from the Fonds de Recherche du Québec - Santé, and the Canadian Institutes of Health Research. MAB also reports personal fees, outside the submitted work, from Pfizer Canada, Shire Pharma, Purdue Pharma, Merck Canada, and Novartis Canada. RLA receives grant support from the NIH, Parkinson's Foundation, and Farmer Family Foundation. RLA serves on the data and safety monitoring boards for Signal-AD (Vaccinex), M-Star (Biohaven), and TANGO (Biogen) trials.
Figures

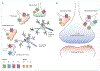
References
-
- Vu TC, Nutt JG, Holford NH. Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment.Br J Clin Pharmacol 2012; 74: 267–83. - PMC - PubMed
-
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 2006; 21: 1123–30. - PubMed
-
- Whitehouse PJ, Hedreen JC, White CL 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol 1983; 13: 243–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

