Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden
- PMID: 35131043
- PMCID: PMC8816388
- DOI: 10.1016/S0140-6736(22)00089-7
Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden
Abstract
Background: Vaccine effectiveness against COVID-19 beyond 6 months remains incompletely understood. We aimed to investigate the effectiveness of COVID-19 vaccination against the risk of infection, hospitalisation, and death during the first 9 months after vaccination for the total population of Sweden.
Methods: This retrospective, total population cohort study was done using data from Swedish nationwide registers. The cohort comprised all individuals vaccinated with two doses of ChAdOx1 nCoV-19, mRNA-1273, or BNT162b2, and matched unvaccinated individuals, with data on vaccinations and infections updated until Oct 4, 2021. Two outcomes were evaluated. The first was SARS-CoV-2 infection of any severity from Jan 12 to Oct 4, 2021. The second was severe COVID-19, defined as hospitalisation for COVID-19 or all-cause 30-day mortality after confirmed infection, from March 15 to Sept 28, 2021.
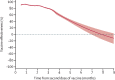
Findings: Between Dec 28, 2020, and Oct 4, 2021, 842 974 individuals were fully vaccinated (two doses), and were matched (1:1) to an equal number of unvaccinated individuals (total study cohort n=1 685 948). For the outcome SARS-CoV-2 infection of any severity, the vaccine effectiveness of BNT162b2 waned progressively over time, from 92% (95% CI 92 to 93; p<0·001) at 15-30 days, to 47% (39 to 55; p<0·001) at 121-180 days, and to 23% (-2 to 41; p=0·07) from day 211 onwards. Waning was slightly slower for mRNA-1273, with a vaccine effectiveness of 96% (94 to 97; p<0·001) at 15-30 days and 59% (18 to 79; p=0·012) from day 181 onwards. Waning was also slightly slower for heterologous ChAdOx1 nCoV-19 plus an mRNA vaccine, for which vaccine effectiveness was 89% (79 to 94; p<0·001) at 15-30 days and 66% (41 to 80; p<0·001) from day 121 onwards. By contrast, vaccine effectiveness for homologous ChAdOx1 nCoV-19 vaccine was 68% (52 to 79; p<0·001) at 15-30 days, with no detectable effectiveness from day 121 onwards (-19% [-98 to 28]; p=0·49). For the outcome of severe COVID-19, vaccine effectiveness waned from 89% (82 to 93; p<0·001) at 15-30 days to 64% (44 to 77; p<0·001) from day 121 onwards. Overall, there was some evidence for lower vaccine effectiveness in men than in women and in older individuals than in younger individuals.
Interpretation: We found progressively waning vaccine effectiveness against SARS-CoV-2 infection of any severity across all subgroups, but the rate of waning differed according to vaccine type. With respect to severe COVID-19, vaccine effectiveness seemed to be better maintained, although some waning became evident after 4 months. The results strengthen the evidence-based rationale for administration of a third vaccine dose as a booster.
Funding: None.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
Comment in
-
Adverse effects of COVID-19 vaccines and measures to prevent them.Virol J. 2022 Jun 5;19(1):100. doi: 10.1186/s12985-022-01831-0. Virol J. 2022. PMID: 35659687 Free PMC article.
References
-
- Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

