Structural and Functional Plasticity in the Dorsolateral Geniculate Nucleus of Mice following Bilateral Enucleation
- PMID: 35131394
- PMCID: PMC8960354
- DOI: 10.1016/j.neuroscience.2022.01.029
Structural and Functional Plasticity in the Dorsolateral Geniculate Nucleus of Mice following Bilateral Enucleation
Abstract
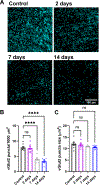
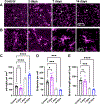
Within the nervous system, plasticity mechanisms attempt to stabilize network activity following disruption by injury, disease, or degeneration. Optic nerve injury and age-related diseases can induce homeostatic-like responses in adulthood. We tested this possibility in the thalamocortical (TC) neurons in the dorsolateral geniculate nucleus (dLGN) using patch-clamp electrophysiology, optogenetics, immunostaining, and single-cell dendritic analysis following loss of visual input via bilateral enucleation. We observed progressive loss of vGlut2-positive retinal terminals in the dLGN indicating degeneration post-enucleation that was coincident with changes in microglial morphology indicative of microglial activation. Consistent with the decline of vGlut2 puncta, we also observed loss of retinogeniculate (RG) synaptic function assessed using optogenetic activation of RG axons while performing whole-cell voltage clamp recordings from TC neurons in brain slices. Surprisingly, we did not detect any significant changes in the frequency of miniature post-synaptic currents (mEPSCs) or corticothalamic feedback synapses. Analysis of TC neuron dendritic structure from single-cell dye fills revealed a gradual loss of dendrites proximal to the soma, where TC neurons receive the bulk of RG inputs. Finally, analysis of action potential firing demonstrated that TC neurons have increased excitability following enucleation, firing more action potentials in response to depolarizing current injections. Our findings show that degeneration of the retinal axons/optic nerve and loss of RG synaptic inputs induces structural and functional changes in TC neurons, consistent with neuronal attempts at compensatory plasticity in the dLGN.
Keywords: bilateral enucleation; dLGN; excitability; intrinsic plasticity; retinogeniculate synapse; thalamocortical.
Copyright © 2022 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

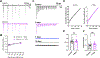




References
-
- Abbott LF, Nelson SB (2000) Synaptic plasticity: taming the beast. Nat Neurosci 3:1178–1183. - PubMed
-
- Arganda-Carreras I, Fernández-González R, Muñoz-Barrutia A, Ortiz-De-Solorzano C (2010) 3D reconstruction of histological sections: Application to mammary gland tissue. Microscopy Research and Technique 73:1019–1029. - PubMed
-
- Beck H, Yaari Y (2008) Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci 9:357–369. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

