Modeling the cellular fate of alpha-synuclein aggregates: A pathway to pathology
- PMID: 35131527
- PMCID: PMC9235864
- DOI: 10.1016/j.conb.2022.01.003
Modeling the cellular fate of alpha-synuclein aggregates: A pathway to pathology
Abstract
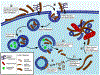
Parkinson's disease is a progressive neurodegenerative disorder that is characterized by pathological protein inclusions that form in the brains of patients, leading to neuron loss and the observed clinical symptoms. These inclusions, containing aggregates of the protein α-Synuclein, spread throughout the brain as the disease progresses. This spreading follows patterns that inform our understanding of the disease. One way to further our understanding of disease progression is to model the discrete steps from when a cell first encounters an aggregate to when those aggregates propagate to new cells. This review will serve to highlight the recent progress made in the effort to better understand the mechanistic steps that determine how this propagation happens at the cellular level.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures
References
-
- Lau LM de, Breteler MM: Epidemiology of Parkinson’s disease. Lancet Neurology 2006, 5:525–535. - PubMed
-
- Marti MJ, Tolosa E, Campdelacreu J: Clinical overview of the synucleinopathies. Movement Disord 2003, 18:21–27. - PubMed
-
- Goedert M, Spillantini MG, Tredici KD, Braak H: 100 years of Lewy pathology. Nat Rev Neurol 2012, 9:13–24. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al.: Mutation in the α-Synuclein Gene Identified in Families with Parkinson’s Disease. Science 1997, 276:2045–2047. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M: α-Synuclein in Lewy bodies. Nature 1997, 388:839–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

