Design and synthesis of novel nitrothiazolacetamide conjugated to different thioquinazolinone derivatives as anti-urease agents
- PMID: 35132095
- PMCID: PMC8821706
- DOI: 10.1038/s41598-022-05736-4
Design and synthesis of novel nitrothiazolacetamide conjugated to different thioquinazolinone derivatives as anti-urease agents
Abstract
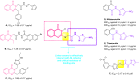
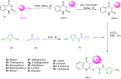
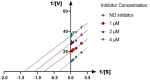
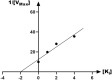
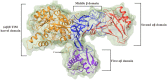
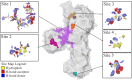
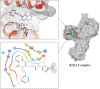
The present article describes the design, synthesis, in vitro urease inhibition, and in silico molecular docking studies of a novel series of nitrothiazolacetamide conjugated to different thioquinazolinones. Fourteen nitrothiazolacetamide bearing thioquinazolinones derivatives (8a-n) were synthesized through the reaction of isatoic anhydride with different amine, followed by reaction with carbon disulfide and KOH in ethanol. The intermediates were then converted into final products by treating them with 2-chloro-N-(5-nitrothiazol-2-yl)acetamide in DMF. All derivatives were then characterized through different spectroscopic techniques (1H, 13C-NMR, MS, and FTIR). In vitro screening of these molecules against urease demonstrated the potent urease inhibitory potential of derivatives with IC50 values ranging between 2.22 ± 0.09 and 8.43 ± 0.61 μM when compared with the standard thiourea (IC50 = 22.50 ± 0.44 μM). Compound 8h as the most potent derivative exhibited an uncompetitive inhibition pattern against urease in the kinetic study. The high anti-ureolytic activity of 8h was confirmed against two urease-positive microorganisms. According to molecular docking study, 8h exhibited several hydrophobic interactions with Lys10, Leu11, Met44, Ala47, Ala85, Phe87, and Pro88 residues plus two hydrogen bound interactions with Thr86. According to the in silico assessment, the ADME-Toxicity and drug-likeness profile of synthesized compounds were in the acceptable range.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Collins CM, D'Orazio SE. Bacterial ureases: Structure, regulation of expression and role in pathogenesis. Mol. Microbiol. 1993;9(5):907–913. - PubMed
-
- Montecucco C, Rappuoli R. Living dangerously: How Helicobacter pylori survives in the human stomach. Nat. Rev. Mol. Cell Biol. 2001;2(6):457–466. - PubMed
-
- Dixon NE, Riddles PW, Gazzola C, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can. J. Biochem. 1980;58(12):1335–1344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

