HR-Bac, a toolbox based on homologous recombination for expression, screening and production of multiprotein complexes using the baculovirus expression system
- PMID: 35132103
- PMCID: PMC8821708
- DOI: 10.1038/s41598-021-04715-5
HR-Bac, a toolbox based on homologous recombination for expression, screening and production of multiprotein complexes using the baculovirus expression system
Erratum in
-
Author Correction: HR-Bac, a toolbox based on homologous recombination for expression, screening and production of multiprotein complexes using the baculovirus expression system.Sci Rep. 2022 Feb 24;12(1):3443. doi: 10.1038/s41598-022-07495-8. Sci Rep. 2022. PMID: 35210550 Free PMC article. No abstract available.
Abstract
The Baculovirus/insect cell expression system is a powerful technology for reconstitution of eukaryotic macromolecular assemblies. Most multigene expression platforms rely on Tn7-mediated transposition for transferring the expression cassette into the baculoviral genome. This allows a rigorous characterization of recombinant bacmids but involves multiple steps, a limitation when many constructs are to be tested. For parallel expression screening and potential high throughput applications, we have established an open source multigene-expression toolbox exploiting homologous recombination, thus reducing the recombinant baculovirus generation to a single-step procedure and shortening the time from cloning to protein production to 2 weeks. The HR-bac toolbox is composed of a set of engineered bacmids expressing a fluorescent marker to monitor virus propagation and a library of transfer vectors. They contain single or dual expression cassettes bearing different affinity tags and their design facilitates the mix and match utilization of expression units from Multibac constructs. The overall cost of virus generation with HR-bac toolbox is relatively low as the preparation of linearized baculoviral DNA only requires standard reagents. Various multiprotein assemblies (nuclear hormone receptor heterodimers, the P-TEFb or the ternary CAK kinase complex associated with the XPD TFIIH subunit) are used as model systems to validate the toolbox presented.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
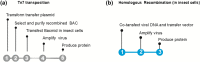


Similar articles
-
A Time and Cost-Effective Pipeline for Expression Screening and Protein Production in Insect Cells Based on the HR-Bac Toolbox to Generate Recombinant Baculoviruses.Methods Mol Biol. 2024;2829:21-48. doi: 10.1007/978-1-0716-3961-0_3. Methods Mol Biol. 2024. PMID: 38951325
-
Protein complex expression by using multigene baculoviral vectors.Nat Methods. 2006 Dec;3(12):1021-32. doi: 10.1038/nmeth983. Nat Methods. 2006. PMID: 17117155
-
GoldenBac: a simple, highly efficient, and widely applicable system for construction of multi-gene expression vectors for use with the baculovirus expression vector system.BMC Biotechnol. 2020 May 12;20(1):26. doi: 10.1186/s12896-020-00616-z. BMC Biotechnol. 2020. PMID: 32398045 Free PMC article.
-
MultiBac: Baculovirus-Mediated Multigene DNA Cargo Delivery in Insect and Mammalian Cells.Viruses. 2019 Feb 26;11(3):198. doi: 10.3390/v11030198. Viruses. 2019. PMID: 30813511 Free PMC article. Review.
-
MultiBac: expanding the research toolbox for multiprotein complexes.Trends Biochem Sci. 2012 Feb;37(2):49-57. doi: 10.1016/j.tibs.2011.10.005. Epub 2011 Dec 7. Trends Biochem Sci. 2012. PMID: 22154230 Free PMC article. Review.
Cited by
-
Application of Baculovirus Expression Vector System (BEVS) in Vaccine Development.Vaccines (Basel). 2023 Jul 8;11(7):1218. doi: 10.3390/vaccines11071218. Vaccines (Basel). 2023. PMID: 37515034 Free PMC article. Review.
-
Equi-MOI ratio for rapid baculovirus-mediated multiprotein co-expression in insect cells integrating selenomethionine for structural studies.FEBS Open Bio. 2025 Apr;15(4):563-572. doi: 10.1002/2211-5463.70025. Epub 2025 Mar 18. FEBS Open Bio. 2025. PMID: 40103323 Free PMC article.
-
Genetic engineering of baculovirus-insect cell system to improve protein production.Front Bioeng Biotechnol. 2022 Sep 20;10:994743. doi: 10.3389/fbioe.2022.994743. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36204465 Free PMC article. Review.
-
Evaluation of Baculoviruses as Gene Therapy Vectors for Brain Cancer.Viruses. 2023 Feb 22;15(3):608. doi: 10.3390/v15030608. Viruses. 2023. PMID: 36992317 Free PMC article.
-
Molecular mechanism of IKK catalytic dimer docking to NF-κB substrates.Nat Commun. 2024 Sep 3;15(1):7692. doi: 10.1038/s41467-024-52076-0. Nat Commun. 2024. PMID: 39227404 Free PMC article.
References
-
- Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J. Virol. 1993;67(8):4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

