Challenges and opportunities for improving access to approved neonatal drugs and devices
- PMID: 35132149
- PMCID: PMC8819193
- DOI: 10.1038/s41372-021-01304-2
Challenges and opportunities for improving access to approved neonatal drugs and devices
Abstract
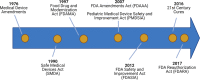
Neonatal drug and device development has lagged behind other patient populations. Oftentimes, providers are using drugs and devices without adequate study of safety and efficacy. Neonates deserve dedicated drug and device development programs, which will require novel approaches and unique collaborations between multiple key stakeholders. Legislative efforts, infrastructure, clinical trial methodology, and international collaborations have all contributed to improvements in neonatal drug and device development, but more work is still needed. Leadership from neonatologists, clinical care providers, and parents is essential to implement needed changes.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources