Cell types of origin of the cell-free transcriptome
- PMID: 35132263
- PMCID: PMC9200634
- DOI: 10.1038/s41587-021-01188-9
Cell types of origin of the cell-free transcriptome
Erratum in
-
Publisher Correction: Cell types of origin of the cell-free transcriptome.Nat Biotechnol. 2022 Jun;40(6):974. doi: 10.1038/s41587-022-01293-3. Nat Biotechnol. 2022. PMID: 35347330 Free PMC article. No abstract available.
Abstract
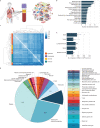
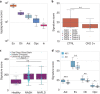
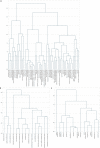
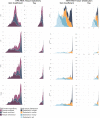
Cell-free RNA from liquid biopsies can be analyzed to determine disease tissue of origin. We extend this concept to identify cell types of origin using the Tabula Sapiens transcriptomic cell atlas as well as individual tissue transcriptomic cell atlases in combination with the Human Protein Atlas RNA consensus dataset. We define cell type signature scores, which allow the inference of cell types that contribute to cell-free RNA for a variety of diseases.
© 2022. The Author(s).
Conflict of interest statement
S.R.Q. is a founder and shareholder of Molecular Stethoscope and Mirvie. M.N.M. is also a shareholder of Mirvie. S.K.V., M.N.M. and S.R.Q. are inventors on a patent application covering the methods and compositions to detect specific cell types using cfRNA submitted by the Chan Zuckerberg Biohub and Stanford University.
Figures








References
-
- Munchel, S. et al. Circulating transcripts in maternal blood reflect a molecular signature of early-onset preeclampsia. Sci. Transl. Med. 12, eaaz0131 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

