Long-term cardiovascular outcomes of COVID-19
- PMID: 35132265
- PMCID: PMC8938267
- DOI: 10.1038/s41591-022-01689-3
Long-term cardiovascular outcomes of COVID-19
Abstract
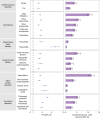
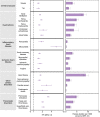
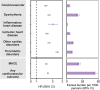
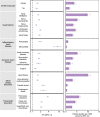
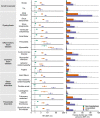
The cardiovascular complications of acute coronavirus disease 2019 (COVID-19) are well described, but the post-acute cardiovascular manifestations of COVID-19 have not yet been comprehensively characterized. Here we used national healthcare databases from the US Department of Veterans Affairs to build a cohort of 153,760 individuals with COVID-19, as well as two sets of control cohorts with 5,637,647 (contemporary controls) and 5,859,411 (historical controls) individuals, to estimate risks and 1-year burdens of a set of pre-specified incident cardiovascular outcomes. We show that, beyond the first 30 d after infection, individuals with COVID-19 are at increased risk of incident cardiovascular disease spanning several categories, including cerebrovascular disorders, dysrhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure and thromboembolic disease. These risks and burdens were evident even among individuals who were not hospitalized during the acute phase of the infection and increased in a graded fashion according to the care setting during the acute phase (non-hospitalized, hospitalized and admitted to intensive care). Our results provide evidence that the risk and 1-year burden of cardiovascular disease in survivors of acute COVID-19 are substantial. Care pathways of those surviving the acute episode of COVID-19 should include attention to cardiovascular health and disease.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures








Comment in
-
Heart-disease risk soars after COVID - even with a mild case.Nature. 2022 Feb;602(7898):560. doi: 10.1038/d41586-022-00403-0. Nature. 2022. PMID: 35145295 No abstract available.
-
Long COVID and cardiovascular disease: a learning health system approach.Nat Rev Cardiol. 2022 May;19(5):287-288. doi: 10.1038/s41569-022-00697-7. Nat Rev Cardiol. 2022. PMID: 35332308 Free PMC article.
-
COVID-19 erhöht kardiovaskuläre Risiken teils dramatisch.MMW Fortschr Med. 2022 May;164(10):20-22. doi: 10.1007/s15006-022-1029-2. MMW Fortschr Med. 2022. PMID: 35585397 Free PMC article. German. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

