The ROS/GRK2/HIF-1 α/NLRP3 Pathway Mediates Pyroptosis of Fibroblast-Like Synoviocytes and the Regulation of Monomer Derivatives of Paeoniflorin
- PMID: 35132350
- PMCID: PMC8817856
- DOI: 10.1155/2022/4566851
The ROS/GRK2/HIF-1 α/NLRP3 Pathway Mediates Pyroptosis of Fibroblast-Like Synoviocytes and the Regulation of Monomer Derivatives of Paeoniflorin
Abstract
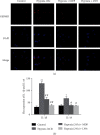
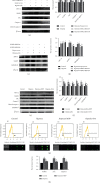
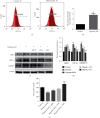
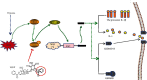
Hypoxia is an important factor in the development of synovitis in rheumatoid arthritis (RA). The previous study of the research group found that monomeric derivatives of paeoniflorin (MDP) can alleviate joint inflammation in adjuvant-induced arthritis (AA) rats by inhibiting macrophage pyroptosis. This study revealed increased levels of hypoxia-inducible factor- (HIF-) 1α and N-terminal p30 fragment of GSDMD (GSDMD-N) in fibroblast-like synoviocytes (FLS) of RA patients and AA rats, while MDP significantly inhibited their expression. Subsequently, FLS were exposed to a hypoxic environment or treated with cobalt ion in vitro. Western blot and immunofluorescence analysis showed increased expression of G protein-coupled receptor kinase 2 (GRK2), HIF-1α, nucleotide-binding oligomerization segment-like receptor family 3 (NLRP3), ASC, caspase-1, cleaved-caspase-1, and GSDMD-N. Electron microscopy revealed FLS pyroptosis after exposure in hypoxia. Next, corresponding shRNAs were transferred into FLS to knock down hypoxia-inducible factor- (HIF-) 1α, and in turn, NLRP3 and western blot results confirmed the same. The enhanced level of GSDMD was reversed under hypoxia by inhibiting NLRP3 expression. Knockdown and overexpression of GRK2 in FLS revealed GRK2 to be a positive regulator of HIF-1α. Levels of GRK2 and HIF-1α were inhibited by eliminating excess reactive oxygen species (ROS). Furthermore, MDP reduced FLS pyroptosis through targeted inhibition of GRK2 phosphorylation. According to these findings, hypoxia induces FLS pyroptosis through the ROS/GRK2/HIF-1α/NLRP3 pathway, while MDP regulates this pathway to reduce FLS pyroptosis.
Copyright © 2022 Zhongyang Hong et al.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures




Similar articles
-
The HIF-1/ BNIP3 pathway mediates mitophagy to inhibit the pyroptosis of fibroblast-like synoviocytes in rheumatoid arthritis.Int Immunopharmacol. 2024 Jan 25;127:111378. doi: 10.1016/j.intimp.2023.111378. Epub 2023 Dec 22. Int Immunopharmacol. 2024. PMID: 38141408
-
The monomer derivative of paeoniflorin inhibits macrophage pyroptosis via regulating TLR4/ NLRP3/ GSDMD signaling pathway in adjuvant arthritis rats.Int Immunopharmacol. 2021 Dec;101(Pt A):108169. doi: 10.1016/j.intimp.2021.108169. Epub 2021 Oct 1. Int Immunopharmacol. 2021. PMID: 34607227
-
SMAD2 inhibits pyroptosis of fibroblast-like synoviocytes and secretion of inflammatory factors via the TGF-β pathway in rheumatoid arthritis.Arthritis Res Ther. 2023 Aug 9;25(1):144. doi: 10.1186/s13075-023-03136-1. Arthritis Res Ther. 2023. PMID: 37559090 Free PMC article.
-
Targeted inhibition of the GRK2/HIF-1α pathway is an effective strategy to alleviate synovial hypoxia and inflammation.Int Immunopharmacol. 2022 Dec;113(Pt A):109271. doi: 10.1016/j.intimp.2022.109271. Epub 2022 Nov 10. Int Immunopharmacol. 2022. PMID: 36461590 Review.
-
SFRP1 Negatively Modulates Pyroptosis of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis: A Review.Front Immunol. 2022 Jun 20;13:903475. doi: 10.3389/fimmu.2022.903475. eCollection 2022. Front Immunol. 2022. PMID: 35795672 Free PMC article. Review.
Cited by
-
Mechanistic and therapeutic insights into the function of NLRP3 inflammasome in sterile arthritis.Front Immunol. 2023 Oct 25;14:1273174. doi: 10.3389/fimmu.2023.1273174. eCollection 2023. Front Immunol. 2023. PMID: 37954594 Free PMC article. Review.
-
Role of reactive oxygen species and mitochondrial damage in rheumatoid arthritis and targeted drugs.Front Immunol. 2023 Feb 9;14:1107670. doi: 10.3389/fimmu.2023.1107670. eCollection 2023. Front Immunol. 2023. PMID: 36845127 Free PMC article. Review.
-
Causal relationship between rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis and risk of prostate cancer: Multivariable and bidirectional Mendelian-randomization analyses.Medicine (Baltimore). 2025 Jan 10;104(2):e41242. doi: 10.1097/MD.0000000000041242. Medicine (Baltimore). 2025. PMID: 39792729 Free PMC article.
-
The Role of Histone Deacetylases in NLRP3 Inflammasomesmediated Epilepsy.Curr Mol Med. 2024;24(8):980-1003. doi: 10.2174/1566524023666230731095431. Curr Mol Med. 2024. PMID: 37519210 Review.
-
New Potentiality of Bioactive Substances: Regulating the NLRP3 Inflammasome in Autoimmune Diseases.Nutrients. 2023 Oct 28;15(21):4584. doi: 10.3390/nu15214584. Nutrients. 2023. PMID: 37960237 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

