The allocation of COVID-19 vaccines and antivirals against emerging SARS-CoV-2 variants of concern in East Asia and Pacific region: A modelling study
- PMID: 35132397
- PMCID: PMC8810205
- DOI: 10.1016/j.lanwpc.2022.100389
The allocation of COVID-19 vaccines and antivirals against emerging SARS-CoV-2 variants of concern in East Asia and Pacific region: A modelling study
Abstract
Background: In view of emerging variants of concern (VOCs), we aimed to evaluate the impact of various allocation strategies of COVID-19 vaccines and antiviral such that the pandemic exit strategy could be tailored to risks and preferences of jurisdictions in the East Asia and Pacific region (EAP) to improve its efficiency and effectiveness.
Methods: Vaccine efficacies were estimated from the titre distributions of 50% plaque reduction neutralization test (PRNT50), assuming that PRNT50 titres of primary vaccination decreased by 2-10 folds due to antibody waning and emergence of VOCs, and an additional dose of vaccine would increase PRNT50 titres by 3- or 9-fold. We then used an existing SARS-CoV-2 transmission model to assess the outcomes of vaccine allocation strategies with and without the use of antivirals for symptomatic patients in Japan, Hong Kong, and Vietnam.
Findings: Increasing primary vaccination coverage was the most important contributing factor in reducing the total and peak number of COVID-19 hospitalisations, especially when population vaccine coverage or vaccine uptake among older adults was low. Providing antivirals to 50% of symptomatic infections only further reduced total and peak hospitalisations by 10-13%. The effectiveness of an additional dose of vaccine was highly dependent on the immune escape potential of VOCs and antibody waning, but less dependent on the boosting efficacy of the additional dose.
Interpretation: Increasing primary vaccination coverage should be prioritised in the design of allocation strategies of COVID-19 vaccines and antivirals against emerging VOCs, such as Omicron, in the EAP region. Heterologous vaccination with any available vaccine as the additional dose could be considered when planning pandemic exit strategies tailored to the circumstances of EAP jurisdictions.
Funding: Health and Medical Research Fund, General Research Fund, AIR@InnoHK.
Keywords: Allocation strategy; Antibody waning; Antiviral; Booster vaccination; COVID-19; Delta variant; Immune escape; Omicron variant; Pandemic exit strategy; SARS-CoV-2; VOC; Vaccination; Variant; Variant of concern.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
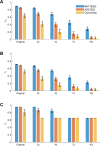
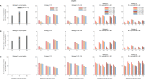
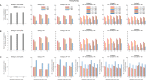

References
-
- Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus pandemic (COVID-19) Our World in Data. 2021
-
- Merck. Merck and Ridgeback's Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study. 2021. https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-ant.... Accessed 13 December 2021.
-
- Merck. Merck and Ridgeback Biotherapeutics Provide Update on Results from MOVe-OUT Study of Molnupiravir, an Investigational Oral Antiviral Medicine, in At Risk Adults With Mild-to-Moderate COVID-19. 2021. https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-u.... Accessed 13 December 2021.
-
- Pfizer. Pfizer's novel covid-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study; 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-n.... Accessed 13 December 2021.
-
- Wadman M. American Association for the Advancement of Science; 2021. Israel's grim warning: Delta can overwhelm shots. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

