Scaffold Hopping from Amodiaquine to Novel Nurr1 Agonist Chemotypes via Microscale Analogue Libraries
- PMID: 35132775
- PMCID: PMC9305750
- DOI: 10.1002/cmdc.202200026
Scaffold Hopping from Amodiaquine to Novel Nurr1 Agonist Chemotypes via Microscale Analogue Libraries
Abstract
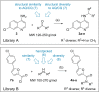
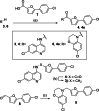
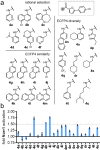
Several lines of evidence suggest the ligand-sensing transcription factor Nurr1 as a promising target to treat neurodegenerative diseases. Nurr1 modulators to validate and exploit this therapeutic potential are rare, however. To identify novel Nurr1 agonist chemotypes, we have employed the Nurr1 activator amodiaquine as template for microscale analogue library synthesis. The first set of analogues was based on the 7-chloroquiolin-4-amine core fragment of amodiaquine and revealed superior N-substituents compared to diethylaminomethylphenol contained in the template. A second library of analogues was subsequently prepared to replace the chloroquinolineamine scaffold. The two sets of analogues enabled a full scaffold hop from amodiaquine to a novel Nurr1 agonist sharing no structural features with the lead but comprising superior potency on Nurr1. Additionally, pharmacophore modeling based on the entire set of active and inactive analogues suggested key features for Nurr1 agonists.
Keywords: NR4A2; neurodegeneration; nuclear receptor-related 1; pharmacophore model; transcription factor.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Development of Nurr1 agonists from amodiaquine by scaffold hopping and fragment growing.Commun Chem. 2024 Jun 29;7(1):149. doi: 10.1038/s42004-024-01224-0. Commun Chem. 2024. PMID: 38951694 Free PMC article.
-
Assessment of NR4A Ligands That Directly Bind and Modulate the Orphan Nuclear Receptor Nurr1.J Med Chem. 2020 Dec 24;63(24):15639-15654. doi: 10.1021/acs.jmedchem.0c00894. Epub 2020 Dec 8. J Med Chem. 2020. PMID: 33289551 Free PMC article.
-
Fragment-like Chloroquinolineamines Activate the Orphan Nuclear Receptor Nurr1 and Elucidate Activation Mechanisms.J Med Chem. 2021 Mar 11;64(5):2659-2668. doi: 10.1021/acs.jmedchem.0c01779. Epub 2021 Feb 25. J Med Chem. 2021. PMID: 33629841
-
Medicinal Chemistry and Chemical Biology of Nurr1 Modulators: An Emerging Strategy in Neurodegeneration.J Med Chem. 2022 Jul 28;65(14):9548-9563. doi: 10.1021/acs.jmedchem.2c00585. Epub 2022 Jul 7. J Med Chem. 2022. PMID: 35797147 Review.
-
Potent synthetic and endogenous ligands for the adopted orphan nuclear receptor Nurr1.Exp Mol Med. 2021 Jan;53(1):19-29. doi: 10.1038/s12276-021-00555-5. Epub 2021 Jan 21. Exp Mol Med. 2021. PMID: 33479411 Free PMC article. Review.
Cited by
-
Development of a Potent Nurr1 Agonist Tool for In Vivo Applications.J Med Chem. 2023 May 11;66(9):6391-6402. doi: 10.1021/acs.jmedchem.3c00415. Epub 2023 Apr 26. J Med Chem. 2023. PMID: 37127285 Free PMC article.
-
Development of Nurr1 agonists from amodiaquine by scaffold hopping and fragment growing.Commun Chem. 2024 Jun 29;7(1):149. doi: 10.1038/s42004-024-01224-0. Commun Chem. 2024. PMID: 38951694 Free PMC article.
-
BRF110, an Orally Active Nurr1-RXRα-Selective Rexinoid, Enhances BDNF Expression without Elevating Triglycerides.J Med Chem. 2025 Feb 27;68(4):4763-4786. doi: 10.1021/acs.jmedchem.4c03046. Epub 2025 Feb 13. J Med Chem. 2025. PMID: 39945195 Free PMC article.
-
Nurr1 Modulation Mediates Neuroprotective Effects of Statins.Adv Sci (Weinh). 2022 Jun;9(18):e2104640. doi: 10.1002/advs.202104640. Epub 2022 Apr 30. Adv Sci (Weinh). 2022. PMID: 35488520 Free PMC article.
-
An optimized Nurr1 agonist provides disease-modifying effects in Parkinson's disease models.Nat Commun. 2023 Jul 18;14(1):4283. doi: 10.1038/s41467-023-39970-9. Nat Commun. 2023. PMID: 37463889 Free PMC article.
References
-
- Wang Z., Benoit G., Liu J., Prasad S., Aarnisalo P., Liu X., Xu H., Walker N. P. C., Perlmann T., Nature 2003, 423, 555–560. - PubMed
-
- Decressac M., Volakakis N., Björklund A., Perlmann T., Nat. Rev. Neurol. 2013, 9, 629–636. - PubMed
-
- Willems S., Zaienne D., Merk D., J. Med. Chem. 2021, 64, 9592–9638. - PubMed
-
- Benoit G., Cooney A., Giguere V., Ingraham H., Lazar M., Muscat G., Perlmann T., Renaud J.-P., Schwabe J., Sladek F., Tsai M.-J., Laudet V., Pharmacol. Rev. 2006, 58, 798–836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

